10-Hydroxycamptothecin
Synonym(s):(S)-10-Hydroxycamptothecin;(S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione
- CAS NO.:19685-09-7
- Empirical Formula: C20H16N2O5
- Molecular Weight: 364.35
- MDL number: MFCD00189425
- EINECS: 805-668-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
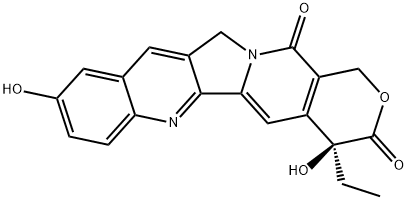
What is 10-Hydroxycamptothecin?
Description
DNA topoisomerases relax supercoiled DNA during replication, transcription, recombination, repair, and chromosome condensation. The relaxation of DNA supercoiling by topoisomerase I at single-
Chemical properties
Yellow Solid
The Uses of 10-Hydroxycamptothecin
A Camptothecin derivative; a topoisomerase inhibitor for cancer therapy
What are the applications of Application
(S)-10-Hydroxycamptothecin is a camptothecin derivative that inhibits DNA topoisomerase I
Definition
ChEBI: 10-Hydroxycamptothecin is a pyranoindolizinoquinoline.
in vitro
10-Hydroxycamptothecin inhibited the growth of BT-20 and MDA-231 cells with IC50 of 34.3nM and 7.27nM, respectively, which was more potent than camptothecin (CPT) with IC50>500nM. 10-Hydroxycamptothecin potently induces the formation of the pBR322 plasmid DNA cleavage complex mediated by human topoisomerase I with an EC50 of 0.35 μM, more than 50-fold more potent than CPT with an EC50 of 18.85 μM. 10-Hydroxycamptothecin treatment caused dose-dependent growth inhibition of human microvascular endothelial cells (HMECs) with IC50 of 0.31 μM and significantly inhibited HMEC migration with IC50 of 0.63 μM. Treatment of HMEC cells with 10-Hydroxycamptothecin also inhibited microtubule formation in a dose-dependent manner with IC50 of 0.96 μM. 10-Hydroxycamptothecin (5-20 nM) significantly inhibits the differentiation of Colo205 cells, arrests the cell cycle in G2 phase, and induces apoptosis through a caspase-3-dependent pathway.
in vivo
In the CAM model, 10-Hydroxycamptothecin treatment inhibited angiogenesis in a concentration-dependent manner, with 95% inhibition at 25 nM, more potent than suramin, which inhibited only 60% of angiogenesis at 125 nM. 10-Hydroxycamptothecin, administered orally at low doses of 2.5-7.5 mg/kg every two days, caused significant growth inhibition in Colo205 xenograft mice, but no acute toxicity. LD50: 104 mg/kg in mice (intraperitoneal injection).
References
[1] vladu b, woynarowski jm, manikumar g, wani mc, wall me, von hoff dd, wadkins rm. 7- and 10-substituted camptothecins: dependence of topoisomerase i-dna cleavable complex formation and stability on the 7- and 10-substituents. mol pharmacol. 2000 feb;57(2):243-51.
[2] xiao d, tan w, li m, ding j. antiangiogenic potential of 10-hydroxycamptothecin. life sci. 2001 aug 24;69(14):1619-28.
[3] ping yh, lee hc, lee jy, wu ph, ho lk, chi cw, lu mf, wang jj. anticancer effects of low-dose 10-hydroxycamptothecin in human colon cancer. oncol rep. 2006 may;15(5):1273-9.
Properties of 10-Hydroxycamptothecin
| Melting point: | 265-270°C |
| Boiling point: | 820.7±65.0 °C(Predicted) |
| Density | 1.60 |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | ≥23.8 mg/mL in DMSO with gentle warming; insoluble in EtOH; insoluble in H2O |
| form | powder to crystal |
| pka | 8.93±0.40(Predicted) |
| color | White to Yellow to Orange |
| CAS DataBase Reference | 19685-09-7(CAS DataBase Reference) |
Safety information for 10-Hydroxycamptothecin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H340:Germ cell mutagenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for 10-Hydroxycamptothecin
| InChIKey | HAWSQZCWOQZXHI-FQEVSTJZSA-N |
10-Hydroxycamptothecin manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
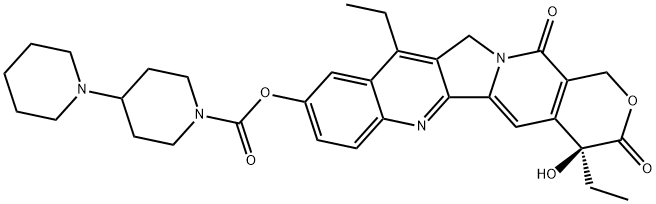
![sodium:(2S)-2-[12-ethyl-8-(hydroxymethyl)-9-oxo-2-(4-piperidin-1-ylpiperidine-1-carbonyl)oxy-11H-indolizino[1,2-b]quinolin-7-yl]-2-hydroxybutanoate](https://img.chemicalbook.in/CAS/20180527/GIF/1329502-92-2.gif)


You may like
-
 19685-09-7 99%View Details
19685-09-7 99%View Details
19685-09-7 -
 10-Hydroxycamptothecin CAS 19685-09-7View Details
10-Hydroxycamptothecin CAS 19685-09-7View Details
19685-09-7 -
 Irinotecan Related Compound A CAS 19685-09-7View Details
Irinotecan Related Compound A CAS 19685-09-7View Details
19685-09-7 -
![19685-09-7 10-Hydroxycamptothecin; (S)-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione) 99.90%](https://img.chemicalbook.in//Content/image/CP5.jpg) 19685-09-7 10-Hydroxycamptothecin; (S)-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione) 99.90%View Details
19685-09-7 10-Hydroxycamptothecin; (S)-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione) 99.90%View Details
19685-09-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
