7-Ethyl-10-hydroxycamptothecin
Synonym(s):(4S)-4,11-Diethyl-4,9-dihydroxy-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)dione;SN-38
- CAS NO.:86639-52-3
- Empirical Formula: C22H20N2O5
- Molecular Weight: 392.4
- MDL number: MFCD06762720
- EINECS: 643-093-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
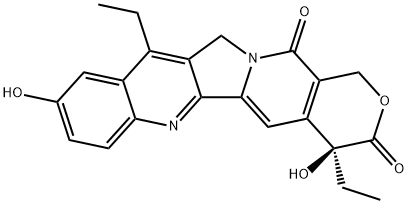
What is 7-Ethyl-10-hydroxycamptothecin?
Description
SN-38 (86639-52-3) is the active metabolite of CPT-11 that inhibits DNA topoisomerase I (IC50 values are 0.74 and 1.9 μM in P388 and Ehrlich cells respectively). Inhibits DNA and RNA synthesis (IC50 values are 0.077 and 1.3 μM respectively) but does not affect protein synthesis. SN-38 displays potent antitumor activity against a range of human tumor cell lines (IC50 values are 3.3, 13, 19 and 22 nM for HCT-116, BEL-7402, HL60 and HeLa cells respectively).
Chemical properties
Light-Yellow Solid
The Uses of 7-Ethyl-10-hydroxycamptothecin
7-ethyl-10-hydroxycamptothecin (SN 38) is a metabolite of Irinotecan, a DNA topoisomerase inhibitor. SN 38 has been used in trials studying the treatment of Cancer, Advanced Solid Tumors, Small Cell Lung Cancer, Metastatic Colorectal Cancer, and Triple Negative Breast Cancer, among others.
What are the applications of Application
SN 38 is the active metabolite of the topoisomerase-I (topo-I) inhibitor Irinotecan (CPT-11), able to inhibit DNA topoisomerase I.
Definition
ChEBI: SN-38 (7-Ethyl-10-hydroxycamptothecin) is a member of the class of pyranoindolizinoquinolines that is (4S)-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione bearing two additional ethyl substituents at positions 4 and 11 as well as two additional hydroxy substituents at positions 4 and 9. It is the active metabolite of irinotecan and is ~1000 times more active than irinotecan itself. It has a role as an apoptosis inducer, an EC 5.99.1.2 (DNA topoisomerase) inhibitor, a drug metabolite and an antineoplastic agent. It is a pyranoindolizinoquinoline, a delta-lactone, a tertiary alcohol and a member of phenols.
Biological Activity
Active metabolite of CPT-11 that inhibits DNA topoisomerase I (IC 50 values are 0.74 and 1.9 μ M in P388 and Ehrlich cells respectively). Inhibits DNA and RNA synthesis (IC 50 values are 0.077 and 1.3 μ M respectively) but does not affect protein synthesis. Displays potent antitumor activity against a range of human tumor cell lines (IC 50 values are 3.3, 13, 19 and 22 nM for HCT-116, BEL-7402, HL60 and HELA cells respectively).
Cytotoxicity
7-Ethyl-10-hydroxycamptothecin has been found to be 200–2000 times more cytotoxic than CPT-11, but has not been used as an anticancer drug due to its poor solubility in pharmaceutically acceptable solvents and low affinity to lipid membranes. SN-38 also undergoes a reversible conversion to an inactive open lactone ring structure at physiological pH.
storage
Store at +4°C
structure and hydrogen bonding
7-Ethyl-10-hydroxycamptothecin belongs to the class of organic compounds known as camptothecins. These are heterocyclic compounds comprising a planar pentacyclic ring structure, that includes a pyrrolo[3,4-beta]-quinoline moiety (rings A, B and C), conjugated pyridone moiety (ring D) and one chiral center at position 20 within the alpha-hydroxy lactone ring with (S) configuration (the E-ring).
References
1) Koizumi et al. (2006), Novel SN-38-incorporating polymeric micelles, NKK012, eradicate vascular endothelial growth factor-secreting bulky tumors; Cancer Res., 66 10048 2) Gao et al. (2005), Synthesis and antitumor activity of the hexacyclic camptothecin derivatives; Bioorg. Med. Chem. Lett., 15 3233 3) Kawato et al. (1991), Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11; Cancer Res., 51 4187
Properties of 7-Ethyl-10-hydroxycamptothecin
| Melting point: | 217 °C |
| Boiling point: | 810.3±65.0 °C(Predicted) |
| Density | 1.51±0.1 g/cm3(Predicted) |
| refractive index | 21.5 ° (C=0.2, THF) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble1mg/mL |
| form | powder |
| pka | 9.13±0.40(Predicted) |
| color | Off-white |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChI | InChI=1S/C22H20N2O5/c1-3-12-13-7-11(25)5-6-17(13)23-19-14(12)9-24-18(19)8-16-15(20(24)26)10-29-21(27)22(16,28)4-2/h5-8,25,28H,3-4,9-10H2,1-2H3/t22-/m0/s1 |
| CAS DataBase Reference | 86639-52-3(CAS DataBase Reference) |
Safety information for 7-Ethyl-10-hydroxycamptothecin
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 7-Ethyl-10-hydroxycamptothecin
| InChIKey | FJHBVJOVLFPMQE-QFIPXVFZSA-N |
| SMILES | N1C2C(=CC(O)=CC=2)C(CC)=C2CN3C(C=12)=CC1[C@](CC)(O)C(=O)OCC=1C3=O |
7-Ethyl-10-hydroxycamptothecin manufacturer
Varanous Labs Pvt Ltd
Avra Laboratories Pvt Ltd
Rivashaa Agrotech Biopharma Pvt. Ltd.
Anand Agencies
ASM Organics
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
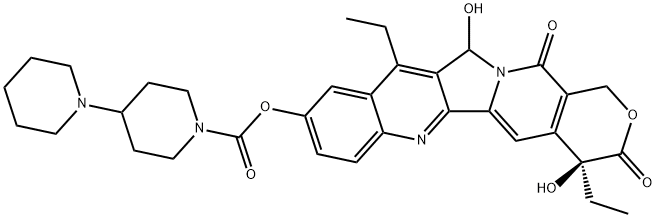
![sodium:(2S)-2-[12-ethyl-8-(hydroxymethyl)-9-oxo-2-(4-piperidin-1-ylpiperidine-1-carbonyl)oxy-11H-indolizino[1,2-b]quinolin-7-yl]-2-hydroxybutanoate](https://img.chemicalbook.in/CAS/20180527/GIF/1329502-92-2.gif)


You may like
-
 86639-52-3 7-ETHYL-10-HYDROXYCAMPTOTHECIN 99%View Details
86639-52-3 7-ETHYL-10-HYDROXYCAMPTOTHECIN 99%View Details
86639-52-3 -
 86639-52-3 99%View Details
86639-52-3 99%View Details
86639-52-3 -
 (S)-7-Ethyl-10-hydroxycamptothecin 98%View Details
(S)-7-Ethyl-10-hydroxycamptothecin 98%View Details
86639-52-3 -
 7-Ethyl-10-hydroxycamptothecin CAS 86639-52-3View Details
7-Ethyl-10-hydroxycamptothecin CAS 86639-52-3View Details
86639-52-3 -
 7-Ethyl-10-hydroxycamptothecin 98%View Details
7-Ethyl-10-hydroxycamptothecin 98%View Details
86639-52-3 -
 (S)-7-Ethyl-10-hydroxycamptothecin 86639-52-3 99%View Details
(S)-7-Ethyl-10-hydroxycamptothecin 86639-52-3 99%View Details
86639-52-3 -
 SN-38 95.00% CAS 86639-52-3View Details
SN-38 95.00% CAS 86639-52-3View Details
86639-52-3 -
 Irinotecan Related Compound B CAS 86639-52-3View Details
Irinotecan Related Compound B CAS 86639-52-3View Details
86639-52-3