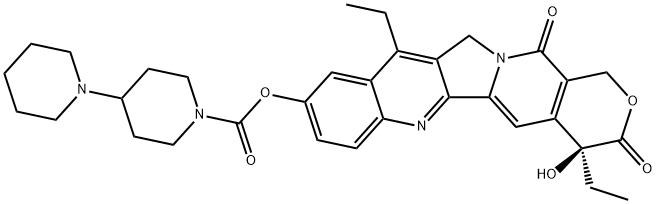
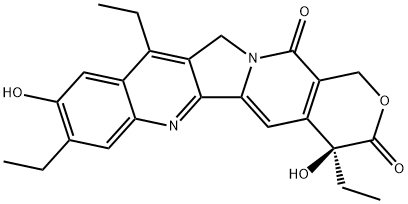
Irinotecan
- CAS NO.:97682-44-5
- Empirical Formula: C33H38N4O6
- Molecular Weight: 586.68
- MDL number: MFCD01862255
- EINECS: 691-567-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
What is Irinotecan?
Absorption
The maximum plasma concentration (Cmax) when a dose of 125 mg/m^2 is given to patients with solid tumours is 1660 ng/mL. The AUC (0-24) is 10,200 ng·h/mL. The Cmax when a dose of 340 mg/m^2 is given to patients with solid tumours is 3392 ng/mL. The AUC (0-24) is 20,604 ng·h/mL.
Toxicity
Gastrointestinal complications, such as nausea, vomiting, abdominal cramping, diarrhea, and infection.
The Uses of Irinotecan
Irinotecan is a topoisomerase I inhibitor for LoVo cells and HT-29 cells with IC50 of 15.8 μM and 5.17 μM, respectively
The Uses of Irinotecan
(+)-Irinotecan is a Topo I inhibitor.
Indications
For the treatment of metastatic colorectal cancer (first-line therapy when administered with 5-fluorouracil and leucovorin). Also used in combination with cisplatin for the treatment of extensive small cell lung cancer. Irinotecan is currently under investigation for the treatment of metastatic or recurrent cervical cancer. Also used in combination with fluorouracil and leucovorin for the treatment of patients with metastatic adenocarcinoma of the pancreas after disease progression following gemcitabine-based therapy.
Background
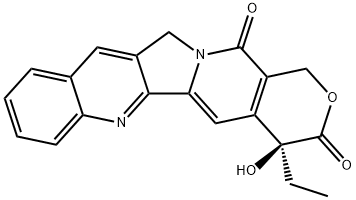
Irinotecan is an antineoplastic enzyme inhibitor primarily used in the treatment of colorectal cancer. It is a derivative of camptothecin that inhibits the action of topoisomerase I. Irinotecan prevents religation of the DNA strand by binding to topoisomerase I-DNA complex, and causes double-strand DNA breakage and cell death. It is a derivative of camptothecin. Irinotecan was approved for the treatment of advanced pancreatic cancer in October, 2015 (irinotecan liposome injection, trade name Onivyde).
What are the applications of Application
(+)-Irinotecan is a Topo I inhibitor
What are the applications of Application
Irinotecan Labeled d10 is a deuterated DNA Topo I inhibitor
Definition
ChEBI: A member of the class of pyranoindolizinoquinolines that is the carbamate ester obtained by formal condensation of the carboxy group of [1,4'-bipiperidine]-1'-carboxylic acid with the phenolic hydroxy group of (4S)-4,11-diethyl-4,9-dihydro y-1H-pyrano[3',4':6,7]indolizino[1,2- hydrochloride]quinoline-3,14-dione. Used (in the form of its hydrochloride salt trihydrate) in combination with fluorouracil and leucovorin, for the treatment of patients with metastatic adenocarcino a of the pancreas after disease progression following gemcitabine-based therapy. It is converted via hydrolysis of the carbamate linkage to its active metabolite, SN-38, which is ~1000 times more active.
brand name
Camptosar (Pharmacia &Upjohn) .
Biological Activity
irinotecan (cpt-11), a prodrug for treating metastatic colorectal cancer, is a topoisomerase i inhibitor for lovo cells and ht-29 cells with ic50 of 15.8 μm and 5.17 μm, respectively [1].in vivo, irinotecan is converted to sn-38, its most active metabolite, by carboxylesterase converting enzyme (cce) [2].
Pharmacokinetics
Irinotecan is an antineoplastic enzyme inhibitor primarily used in the treatment of colorectal cancer. Irinotecan is a semisynthetic derivative of camptothecin. Camptothecins interact specifically with topoisomerase I, an enzyme in the cell nucleus that regulates DNA topology and facilitates nuclear processes such as DNA replication, recombination, and repair. During these processes, topoisomerase I relieves torsional strain in DNA by inducing reversible single-strand breaks, allowing single DNA strands to pass through the break. The 3'-DNA terminus of the broken DNA strands bind covalently with the topoisomerase enzyme to form a catalytic intermediate called a cleavable complex. After the DNA is sufficiently relaxed and the strand passage reaction is complete, DNA topoisomerase reattaches the broken DNA strands to form the chemically unaltered topoisomers that allow transcription to proceed. Irinotecan and its active metabolite SN-38 bind to the topoisomerase I-DNA complex and prevent religation of these single-strand breaks. Current research suggests that the cytotoxicity of irinotecan is due to double-strand DNA damage produced during DNA synthesis when replication enzymes interact with the ternary complex formed by topoisomerase I, DNA, and either Irinotecan or SN-38. Mammalian cells cannot efficiently repair these double-strand breaks. The precise contribution of SN-38 to the activity of irinotecan in humans is not known. Irinotecan is cell cycle phase-specific (S-phase).
in vitro
irinotecan induced similar amounts of cleavable complexes in lovocells and ht-29 cell lines with the ic50 of 15.8 μm and 5.17 μm, respectively [1].after addition of 157 mm irinotecan to plasma, sn-38 concentration showed linear increase during the first 60-min period, followed by a plateau.in the first 60 min, mean and standard deviation of the conversion rate were 515.9 ± 50.1 pmol/ml/h (n = 69), with a coefficient of variation of 0.097 [2]. irinotecan (cpt-11) was significantly more active in sclc than in nsclccelllines (p = 0.0036). ce activity appeared to be associated with higher sensitivity to cpt-11 in human lung cancercelllines and may partly explain the difference in the in vitro sensitivity to cpt-11 between sclc and nsclccells [3].in vitro, the sensitivity to cpt-11 and sn-38 was highest in ls174t and colo 320cells, intermediate in sw1398cellsand lowest in colo 205 and widr cells. the activity of sn-38 was 130 to 570 times than cpt-11[4].
in vivo
in colo 320 xenografts, irinotecan induced a maximum growth inhibition of 92% [4].a single dose of irinotecan significantly increased amounts of topoisomerase i covalently bound to dna in stomach, duodenum, colon and liver. concomitantly, the irinotecan-treated group exihibited significantly higher amounts of dna strand breaks in colon mucosa cells compared to the control group [5].
Metabolism
Hepatic. The metabolic conversion of irinotecan to the active metabolite SN-38 is mediated by carboxylesterase enzymes and primarily occurs in the liver. SN-38 is subsequently conjugated predominantly by the enzyme UDP-glucuronosyl transferase 1A1 (UGT1A1) to form a glucuronide metabolite.
References
[1]. tobin p, clarke s, seale j p, et al. the in vitro metabolism of irinotecan (cpt‐11) by carboxylesterase and β‐glucuronidase in human colorectal tumours[j]. british journal of clinical pharmacology, 2006, 62(1): 122-129.
[2]. shingyoji m, takiguchi y, watanabe‐uruma r, et al. in vitro conversion of irinotecan to sn‐38 in human plasma[j]. cancer science, 2004, 95(6): 537-540.
[3]. van ark-otte j, kedde m a, van der vijgh w j, et al. determinants of cpt-11 and sn-38 activities in human lung cancer cells[j]. british journal of cancer, 1998, 77(12): 2171.
[4]. jansen w j m, zwart b, hulscher s t m, et al. cpt-11 in human colon-cancer cell lines and xenografts: characterization of cellular sensitivity determinants[j]. international journal of cancer, 1997, 70(3): 335-340.
[5]. na y s, jung k a, kim s m, et al. the histone deacetylase inhibitor pxd101 increases the efficacy of irinotecan in in vitro and in vivo colon cancer models[j]. cancer chemotherapy and pharmacology, 2011, 68(2): 389-398.
Properties of Irinotecan
| Melting point: | 222-223° |
| Boiling point: | 873.4±65.0 °C(Predicted) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Acetonitrile (Slightly, Heated, Sonicated), DMSO (Slightly), Methanol (Slightly) |
| pka | 11.20±0.20(Predicted) |
| form | Solid |
| color | White to Brown |
| CAS DataBase Reference | 97682-44-5(CAS DataBase Reference) |
Safety information for Irinotecan
Computed Descriptors for Irinotecan
Irinotecan manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![sodium:(2S)-2-[12-ethyl-8-(hydroxymethyl)-9-oxo-2-(4-piperidin-1-ylpiperidine-1-carbonyl)oxy-11H-indolizino[1,2-b]quinolin-7-yl]-2-hydroxybutanoate](https://img.chemicalbook.in/CAS/20180527/GIF/1329502-92-2.gif)


You may like
-
 97682-44-5 Irinotecan 98%View Details
97682-44-5 Irinotecan 98%View Details
97682-44-5 -
 97682-44-5 99%View Details
97682-44-5 99%View Details
97682-44-5 -
 Irinotecan 98%View Details
Irinotecan 98%View Details
97682-44-5 -
 Irinotecan 97682-44-5 98%View Details
Irinotecan 97682-44-5 98%View Details
97682-44-5 -
 97682-44-5 Irinotecan 98%View Details
97682-44-5 Irinotecan 98%View Details
97682-44-5 -
 Irinotecan 98% (HPLC) CAS 97682-44-5View Details
Irinotecan 98% (HPLC) CAS 97682-44-5View Details
97682-44-5 -
 Irinotecan 99.50%View Details
Irinotecan 99.50%View Details
97682-44-5 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
