1-HYDROXYCLOBUT-1-ENE-3,4-DIONE SODIUM SALT
Synonym(s):1-Hydroxycyclobut-1-ene-3,4-dione;1-Hydroxycyclobut-1-ene-3,4-dione sodium salt;Semisquaric acid sodium salt
- CAS NO.:71376-34-6
- Empirical Formula: C4H3NaO3
- Molecular Weight: 122.05
- MDL number: MFCD00056566
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
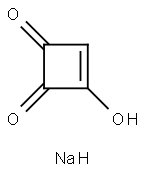
What is 1-HYDROXYCLOBUT-1-ENE-3,4-DIONE SODIUM SALT?
Chemical properties
Solid
The Uses of 1-HYDROXYCLOBUT-1-ENE-3,4-DIONE SODIUM SALT
Moniliformin is a potent, water-soluble mycotoxin produced by several species of Fusarium. Comparative toxicity studies of moniliformin in chicken cell lines revealed no toxicity to chondrocytes and macrophages, but toxicity to splenocytes, cardiac and skeletal myocytes. Moniliformin selectively inhibits mitochondrial oxidization of pyruvate and α-ketoglutarate, however the mode of action is not yet completely resolved.
The Uses of 1-HYDROXYCLOBUT-1-ENE-3,4-DIONE SODIUM SALT
Moniliformin sodium salt from Fusarium proliferatum has been used as a mycotoxin standard:
- to test its acute oral toxicity in mice
- to test its subacute toxic effects in rats
- in characterizing mycotoxins from Aspergillus
What are the applications of Application
Moniliformin sodium salt is a potentially useful mycotoxin isolate from Fusarium moniliforme
General Description
Moniliformin (MON), a mycotoxin and small ionic molecule, is present in various Fusarium species including Fusarium avenaceum, Fusarium subglutinans and Fusarium proliferatum. It is present as sodium or potassium salt of semisquaric acid naturally. MON is also present in maize and small-grain cereals.
Biological Activity
moniliformin induces mitotic arrest at the metaphase stage.mitosis is a part of the cell cycle when replicated chromosomes are separated into two new nuclei. the process of mitosis is divided into stages corresponding to the completion of one set of activities and the start of the next.
Biochem/physiol Actions
Moniliformin (MON) is implicated for its toxic potential and may lead to respiratory distress and progressive muscular weakness in rats. It inhibits the tricarboxylic acid (TCA) cycle oxidation step. By acting as a pyruvate substrate, MON effectively inhibits thiamine pyrophosphate cofactor dependant enzymes and blocks the gluconeogenesis pathway. ) Furthermore, MON also inhibits glutathione peroxidase and glutathione reductase leading to oxidative stress in myoblast.
in vitro
moniliformin, first isolated as a mycotoxin from fusarium moniliforme, was found to be phytotoxic and arrests mitosis of maize root meristematic cells at the metaphase stage. the mitotic spindle could be disrupted by the treatment of moniliformin, but no direct effect on tubulin had been observed [1].
in vivo
a previous study was conducted on rat heart to in situ determine the myocardial toxicity of moniliformin, originally isolated from mouldy corn and soil samples in the keshan disease prevalent area in china. results showed that perfusion of moniliformin 10-7 mol/liter in isolated heart decreased myocardial contractile force by 52%. intravenous injection of moniliformin at 1/6 and 1/4 ld50 could markedly inhibit cardiac hemodynamic variables associated with myocardial contractile function. moreover, moniliformin was able to decrease +/- lv dp/dt max by 52%, and induce ventricular arrhythmia. these findings indicated that moniliformin was toxic to mammalian heart and might be an important factor relative to keshan disease [2].
References
[1] duke, s. o. and dayan, f.e. modes of action of microbially-produced phytotoxins. toxins (basel) 3(8), 1038-1064 (2011).
[2] fan ll, li j, sun lh. effect of moniliformin on myocardial contractility in rats. biomed environ sci. 1991 sep;4(3):290-4.
Properties of 1-HYDROXYCLOBUT-1-ENE-3,4-DIONE SODIUM SALT
| Flash point: | 2℃ |
| storage temp. | 2-8°C |
| solubility | ≤10mg/ml in H2O |
| form | Light yellow powder. |
| color | Light yellow to yellow |
Safety information for 1-HYDROXYCLOBUT-1-ENE-3,4-DIONE SODIUM SALT
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for 1-HYDROXYCLOBUT-1-ENE-3,4-DIONE SODIUM SALT
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Moniliformin sodium salt from Fusarium proliferatum CAS 71376-34-6View Details
Moniliformin sodium salt from Fusarium proliferatum CAS 71376-34-6View Details
71376-34-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8