1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
Synonym(s):EDC hydrochloride;EDC;EDAC;WSC hydrochloride;N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- CAS NO.:25952-53-8
- Empirical Formula: C8H18ClN3
- Molecular Weight: 191.7
- MDL number: MFCD00044916
- EINECS: 247-361-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-30 18:55:25
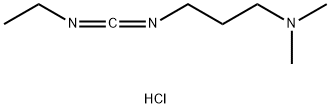
What is 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride?
Chemical properties
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is a white crystalline powder. It is commonly used as an activating reagent in amide synthesis to activate carboxyl groups. It can also activate phosphate groups, cross-link proteins and nucleic acids, and produce immunocouplers.
What are the applications of Application
EDC hydrochloride is a water soluble carboxyl activating carbodiimide reagent, used for amide bond formation.
Biochem/physiol Actions
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride(25952-53-8) is a water soluble condensing reagent. EDAC is generally utilized as a carboxyl activating agent for amide bonding with primary amines. Additionally, it reacts with phosphate groups. It has been utilized in peptide synthesis, crosslinking proteins to nucleic acids as well as preparation of immunoconjugates.
Mechanism of action
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is transformed into the corresponding urea during coupling reactions. It has the advantage over DCU in that it can be removed from the reaction mixture by extraction or be washed out from solid phase synthesis applications. However, the O-acylisourea can rearrange irreversibly to an N-acyl urea and racemise the α-carbon of the amino acid via the formation of an oxazol-4(5H)-one [azlactone]. N-Acylurea formation and racemisation may be reduced by using intermediate nucleophiles, which convert the O-acylurea to an activated ester containing the nucleophile. The mechanism of amide bond formation applies both to EDC and EDC.HCl.
Synthesis
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride can be used for the synthesis of amides.It is used as a coupling agent in the synthesis of esters from carboxylic acids using dimethylaminopyridine as the catalyst.
Preparation method of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride(CN104193654A)
Description
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC or EDAC) is a zero-length crosslinking agent to couple carboxyl groups to primary amines. This crosslinker has been used in diverse applications such as forming amide bonds in peptide synthesis, attaching haptens to carrier proteins to form immunogens, labeling nucleic acids through 5' phosphate groups, and creating amine-reactive NHS-esters of biomolecules. EDC reacts with a carboxyl to form an amine-reactive O-acylisourea intermediate. If this intermediate does not encounter an amine, it will hydrolyze and regenerate the carboxyl group. In the presence of N-hydroxysulfosuccinimide (Sulfo-NHS), EDC can be used to convert carboxyl groups to amine-reactive Sulfo-NHS esters. This is accomplished by mixing the EDC with a carboxyl-containing molecule and adding Sulfo-NHS.
The Uses of 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
EDC hydrochloride is a water-soluble derivative of carbodiimide useful for conjugating haptens to proteins and polypeptides. Used to modify NMDA receptors and as a condensing agent in peptide synthesis.
Properties of 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
| Melting point: | 110-115 °C(lit.) |
| Density | 0.877 g/mL at 20 °C(lit.) |
| vapor pressure | 0.002Pa at 20℃ |
| refractive index | n |
| storage temp. | -20°C |
| solubility | H2O: soluble1 gm/10 ml, clear to very slightly hazy, colorless to very faintly yellow |
| form | Crystalline Powder |
| color | White to off-white |
| Water Solubility | Soluble |
| Sensitive | Hygroscopic |
| BRN | 5764110 |
| Stability: | Stable, but sensitive to moisture. Incompatible with strong acids, strong oxidizing agents, moisture. |
| CAS DataBase Reference | 25952-53-8(CAS DataBase Reference) |
| EPA Substance Registry System | 1,3-Propanediamine, N'-(ethylcarbonimidoyl)-N,N-dimethyl-, monohydrochloride (25952-53-8) |
Safety information for 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal H315:Skin corrosion/irritation H317:Sensitisation, Skin H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
| InChIKey | FPQQSJJWHUJYPU-UHFFFAOYSA-N |
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride manufacturer
Clickchem Research LLP
BTC Pharmaceuticals Technology India
ASM Organics
CLEARSYNTH LABS LTD.
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 High Quality 1- (3-Dimethylaminopropyl) -3-Ethylcarbodiimide Hydrochloride EDC HClView Details
High Quality 1- (3-Dimethylaminopropyl) -3-Ethylcarbodiimide Hydrochloride EDC HClView Details
25952-53-8 -
 N-Ethyl-N'-(3- dimethylaminopropyl)carbodiimide hydrochloride, 98% 25952-53-8 98%View Details
N-Ethyl-N'-(3- dimethylaminopropyl)carbodiimide hydrochloride, 98% 25952-53-8 98%View Details
25952-53-8 -
 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 99% CAS 25952-53-8View Details
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 99% CAS 25952-53-8View Details
25952-53-8 -
 1-(3-Dimethylaminopropyl)-3-Ethyl Carbodiimide Hydrochloride (EDC.HCl CAS 25952-53-8View Details
1-(3-Dimethylaminopropyl)-3-Ethyl Carbodiimide Hydrochloride (EDC.HCl CAS 25952-53-8View Details
25952-53-8 -
 EDAC.HCl;1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 99% CAS 25952-53-8View Details
EDAC.HCl;1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 99% CAS 25952-53-8View Details
25952-53-8 -
 1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide Hydrochloride, Cas No:25952-53-8View Details
1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide Hydrochloride, Cas No:25952-53-8View Details
25952-53-8 -
 EDC HCL, 1 Kg BagView Details
EDC HCL, 1 Kg BagView Details
25952-53-8 -
 EDC-HCL (25952-53-8)View Details
EDC-HCL (25952-53-8)View Details
25952-53-8
