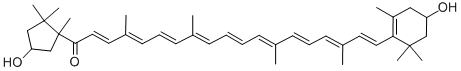
Lycopene
Synonym(s):ψ,ψ-Carotene;2,6,10,14,19,23,27,31-Octamethyl-dotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene;E160d;Lycopene;Lycopene, dispersion
- CAS NO.:502-65-8
- Empirical Formula: C40H56
- Molecular Weight: 536.87
- MDL number: MFCD00017350
- EINECS: 207-949-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18

What is Lycopene?
Description
Lycopene is a red-
Description
August 3 is National Watermelon Day, and so this week’s molecules have a watermelon flavor.
Lycopene is the source of the red color in several fruits, notably watermelon (Citrullus lanatus?var.?lanatus) and tomato (Solanum lycopersicum). The pigment, also known as ψ,ψ-carotene, is a linear polyolefin that contains eight isoprene units. It has 13 double bonds, all in the?E-configuration.
In 1910, Swiss chemists Richard M. Willst?tter and Heinrich H. Escher isolated lycopene from tomatoes and determined its structure. But not until 1950 did another Swiss chemist, Paul Karrer, and his co-workers synthesize it.
Lycopene is an antioxidant that may have some health benefits, particularly against cancer and cardiovascular diseases. But agencies such as the European Food Safety Authority and the US Food and Drug Administration are not convinced; on the basis of current evidence, they have refused to label lycopene as a beneficial drug.
Forchlorfenuron, formally 1-(2-chloropyridin-4-yl)-3-phenylurea (CPPU), is a growth regulator that increases the size (and therefore the value) of such fruits as apple, cherry, and kiwi. It’s been used on watermelon too; but at least once, the results were unexpected.
In China in 2011, about 20 farmers applied too much CPPU on their watermelon crops too late in the growing season. The weather conditions were ideal, so much so that the watermelons grew too fast and exploded. The watermelon fragments, about 46 hectares worth, were used to feed fish and pigs.
Chemical properties
Lycopene is a white to pale-yellow solid; balsam oriental aroma. Lycopene extract from tomato is a dark-red viscous liquid. It is freely soluble in ethyl acetate and n-hexane, partially soluble in ethanol and acetone, and insoluble in water. A solution in n-hexane shows an absorption maximum at approximately 472nm.
Lycopene (from the Greek word lykopersikon, meaning tomato) is a bright red carotene and carotenoid pigment. The natural resources are red fruits and vegetables, such as tomatoes, pink grapefruit, watermelon, and apricots. After absorbing from the stomach, lycopene is transported in the blood and accumulates in the liver, adrenal glands, and testes. Lycopene has been used to prevent carcinogenesis, cardiovascular diseases and aging.

From a chemistry perspective, lycopene is a symmetrical tetraterpene assembled from 8 isoprene units, containing 11 conjugated and 2 non-conjugated double bonds between carbon atoms. Lycopene is a member of the carotenoid family, and the predominant source in the human diet comes from tomato and tomato-based products. The antioxidant capacity of tomato strongly depends on the content and bioavailability of lycopene in the fruit. There is strong correlation between lycopene content in tomatoes and antioxidant capacity.
Occurrence
Lycopene is a carotenoid that occurs naturally in tomatoes.
The Uses of Lycopene
Lycopene extract from tomato is intended for use as a food colour. It provides the similar colour shades, ranging from yellow to red, as do the natural and synthetic lycopenes. Lycopene extract from tomato is also used as a food/dietary supplement in products where the presence of lycopene provides a specific value (e.g., antioxidant or other claimed health benefits). The product may also be used as an antioxidant in food supplements.
Lycopene extract from tomato is intended for use in the following food categories: baked goods, breakfast cereals, dairy products including frozen dairy desserts, dairy product analogues, spreads, bottled water, carbonated beverages, fruit and vegetable juices, soybean beverages, candy, soups, salad dressings, and other foods and beverages.
The Uses of Lycopene
Carotenoid antioxidant occurring in ripe fruit, especially in tomatoes.
The Uses of Lycopene
Lycopene has been used:
- in high performance liquid chromatography (HPLC) to determine its concentration in liver, kidney and lung tissue
- to induce urokinase plasminogen activator receptor (uPAR) IN prostate cancer cell line
- in Raman chemical imaging system to detect and visualize its internal distribution
Background
Lycopene is a naturally occuring red carotenoid pigment that is responsible in red to pink colors seen in tomatoes, pink grapefruit, and other foods . Having a chemical formula of C40H56, lycopene is a tetraterpene assembled from eight isoprene units that are solely composed of carbon and hydrogen. Lycophene may undergo extensive isomerization that allows 1056 theoretical cis-trans configurations; however the all-trans configuration of lycopene is the most predominant isomer found in foods that gives the red hue. Lycopene is a non-essential human nutrient that is classified as a non-provitamin A carotenoid pigment since it lacks a terminal beta ionone ring and does not mediate vitamin A activity. However lycophene is a potent antioxidant molecule that scavenges reactive oxygen species (ROS) singlet oxygen. Tomato lycopene extract is used as a color additive in food products.
What are the applications of Application
Lycopene is a highly conjugated antioxidant carotenoid compound
Definition
ChEBI: An acyclic carotene commonly obtained from tomatoes and other red fruits.
Production Methods
Lycopene extract from tomato is produced from a tomato variety with high lycopene content, within the range of 150 to 250 mg/kg. This particular variety is not generally marketed for direct consumption, but is used primarily in the production of this lycopene extract. The extract is produced by crushing tomatoes into crude tomato juice that is then separated into serum and pulp. The tomato pulp is then extracted with ethyl acetate. The final product is obtained after solvent removal by evaporation under vacuum at 40-60°C.
Aroma threshold values
Medium strength odor, balsamic type; recommend smelling in a 1.0% solution or less.
General Description
Lycopene is a naturally occurring red pigment, which belongs to the family of carotenoids. It is found in tomatoes, watermelon and papaya. Lycopene has antioxidant property.
Biological Activity
Lycopene may act as an inhibitor of tumor cells. In one study, lycopene was shown to inhibit PDGF-BB-induced signalling and cell migration in human cultured skin fibroblasts (Wu et al., 2007). Trapping of PDGF by lycopene compromised melanoma-induced fibroblast migration and attenuated signalling transduction in fibroblasts (Wu et al., 2007). In functional studies, lycopene inhibited melanoma-induced fibroblast migration in a noncontact coculture system and attenuated signalling in fibroblasts simulated by melanoma-derived conditioned medium (Chiang et al., 2007).
Biochem/physiol Actions
Antioxidant micronutrient of tomatoes associated with decreased risk for cancer and cardiovascular disease. Enhances gap juction communication between cells via upregulation of connexin 43 and reduces proliferation of cancer cells in culture. Inhibits cholesterol synthesis and enhances low-density lipoprotein degradation.
Mechanism of action
The biological activities of carotenoids such as βcarotene are related in general to their ability to form vitamin A within the body.Since lycopene lacks the β-ionone ring structure, it cannot form vitamin A.Its biological effects in humans have therefore been attributed to mechanisms other than vitamin A. Two major hypotheses have been proposed to explain the anticarcinogenic and antiatherogenic activities of lycopene: nonoxidative and oxidative mechanisms.
Among the nonoxidative mechanisms, the anticarcinogenic effects of lycopene have been suggested to be due to regulation of gap-junction communication in mouse embryo fibroblast cells.Lycopene is hypothesized to suppress carcinogen-induced phosphorylation of regulatory proteins such as p53 and Rb antioncogenes and stop cell division at the G0–G1 cell cycle phase.Astorg and colleagues proposed that lycopene-induced modulation of the liver metabolizing enzyme, cytochrome P4502E1, was the underlying mechanism of protection against carcinogen-induced preneoplastic lesions in the rat liver. Preliminary in vitro evidence also indicates that lycopene reduces cellular proliferation induced by insulin-like growth factors, which are potent mitogens, in various cancer cell lines.Regulation of intrathymic T-cell differentiation (immunomodulation) was suggested to be the mechanism for suppression of mammary tumour growth by lycopene treatments in SHN retired mice.Lycopene also has been shown to act as a hypocholesterolemic agent by inhibiting HMG–CoA (3-hydroxy-3-methylglutaryl–coenzyme A) reductase.
Lycopene has been hypothesized to prevent carcinogenesis and atherogenesis by protecting critical cellular biomolecules, including lipids, lipoproteins, proteins and DNA.In healthy human subjects, lycopene- or tomatofree diets resulted in loss of lycopene and increased lipid oxidation,whereas dietary supplementation with lycopene for 1 week increased serum lycopene levels and reduced endogenous levels of oxidation of lipids, proteins, lipoproteins and DNA.Patients with prostate cancer were found to have low levels of lycopene and high levels of oxidation of serum lipids and proteins.
Mechanism of action
Lycopene is a red carotenoid compound found in pink grapefruit, papaya, wolfberry, goji, and tomatoes Dietary supplementation with tomato-based products appears to lower biomarkers of oxidative stress and carcinogenesis. Limited available evidence from small human intervention studies indicate that lycopene supplementation for 10–12 weeks may decrease UV-induced erythema. Although the bioavailability of lycopene in raw tomatoes is low due to tight binding with indigestible fiber, lycopene can be released from the food matrix through heating and food processing. The effect of topical lycopene is not well characterized. An in vivo study using SKH-1 hairless mice found that topical lycopene reduced the activity of ornithine decarboxylase (ODC) and myeloperoxidase (MPO), enzymes that have been implicated in the carcinogenic and acute inflammatory effect of UVB irradiation.
Anticancer Research
Lycopene is a naturally occurring chemical that manifests as a red pigment contained in common foods such as tomatoes, pink grapefruits, guava, and watermelon (Giovannucci 1999). This is a very strong antioxidant that has been found to prevent and even reverse the progression of prostate cancer, as well as treating benign prostatic hyperplasia. In a recent study, 30 mg a day of lycopene showed curative results in prostate cancer. For best results, supplements are recommended alongside eating and drinking plenty of lycopene-containing food and juices (Jatoi et al. 2007). Earlier research showed that taking a specific combination of lycopene, selenium, and saw palmetto by mouth for 8 weeks reduced pain in men with prostate swelling and pelvic pain more significantly than saw palmetto alone (Feifer et al. 2002).Lycopene shows anticancer activity against prostate, endometrial, breast, and colon carcinomas. It inhibits human cancer cell proliferation by activation of cancer-preventive enzymes like phase II detoxification enzymes, by suppression of insulin-like growth factor-I-stimulated growth (Wang et al. 2012). It also activates antioxidant enzymes like GST, GSH, and GPx and protects from oxidative stress caused by carcinogens. It alters PI3K/AKT pathway and ERK and Bcl-2 signaling in pancreatic and gastric carcinoma cells, respectively (Singh et al. 2016b).
Metabolism
Not Available
Purification Methods
Crystallise lycopene from CS2/MeOH, diethyl ether/pet ether, or acetone/pet ether. Also purify it by column chromatography on deactivated alumina, CaCO3, calcium hydroxide or magnesia. It is oxygen sensitive and is stored in the dark, in an inert atmosphere. Also purified like -Carotene. [Beilstein 1 III 1076, 1 IV 1165.]
Properties of Lycopene
| Melting point: | 172-173°C |
| Boiling point: | 644.94°C (rough estimate) |
| Density | 0.9380 (estimate) |
| refractive index | 1.5630 (estimate) |
| FEMA | 4110 | TOMATO LYCOPENE |
| storage temp. | -70°C |
| solubility | Benzene (Slightly), Chloroform (Sparingly), Ethyl Acetate (Very Slightly), Metha |
| form | powder |
| color | Red to Very Dark Red |
| Odor | balsam oriental |
| Stability: | Heat sensitive - store at -70 C. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 502-65-8(CAS DataBase Reference) |
Safety information for Lycopene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Lycopene
| InChIKey | OAIJSZIZWZSQBC-BOJOQWLHSA-N |
Lycopene manufacturer
Shree Sai Biotech
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Lycopene 99%View Details
Lycopene 99%View Details -
 Lycopene CAS 502-65-8View Details
Lycopene CAS 502-65-8View Details
502-65-8 -
 Lycopene, Grade Standard: Food Grade, Packaging Size: 5 KGView Details
Lycopene, Grade Standard: Food Grade, Packaging Size: 5 KGView Details
502-65-8 -
 Lycopene CAS 502-65-8View Details
Lycopene CAS 502-65-8View Details
502-65-8 -
 Lycopene Powder, Grade Standard: Food GradeView Details
Lycopene Powder, Grade Standard: Food GradeView Details
502-65-8 -
 LycopeneView Details
LycopeneView Details
502-65-8 -
 Lycopene 6% & 10% (Oil / Powder)View Details
Lycopene 6% & 10% (Oil / Powder)View Details
502-65-8 -
 LycopeneView Details
LycopeneView Details
502-65-8
