(-)-Blebbistatin
Synonym(s):(-)-Blebbistatin - CAS 856925-71-8 - Calbiochem;1-Phenyl-1,2,3,4-tetrahydro-4-hydroxypyrrolo[2.3-b]-7-methylquinolin-4-one
- CAS NO.:856925-71-8
- Empirical Formula: C18H16N2O2
- Molecular Weight: 292.33
- MDL number: MFCD08460907
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
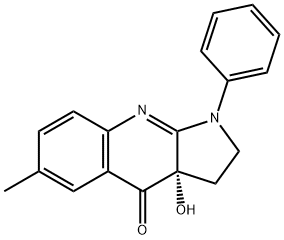
What is (-)-Blebbistatin?
Chemical properties
Bright Yellow Solid
The Uses of (-)-Blebbistatin
Blebbistatin blocked myosin II-dependent cell processes. It blocks cell blebbing rapidly and reversibly. Also rapidly disrupted directed cell migration and cytokinesis in vertebrate cell. It blocks both blebbing and cytokinesis at 50-100μM. [a]D= -103
The Uses of (-)-Blebbistatin
(?)-Blebbistatin has been used to investigate the effect on cardiac contractility and effect of thrombin on actomyosin contractility
What are the applications of Application
(S)-(?)-Blebbistatin is an inhibitor of myosin II
Definition
ChEBI: (S)-blebbistatin is the (S)-enantiomer of blebbistatin. It is a blebbistatin and a tertiary alpha-hydroxy ketone.
General Description
(?)-Blebbistatin, a myosin II ATPase inhibitor, does not affect other members of myosin superfamily like I, V and X. Blebbistatin is highly light sensitive, and photobleaching can reverse its effect completely. Blebbistatin is a commonly used as excitation-contraction uncoupling agent in cardiovascular physiology. Blebbistatin controls the inhibitory effect of thrombin on intercellular Ca2+ wave propagation. Blebbistatin reduces cardiac contractility by stabilizing the OFF state of the thick filament.
Biological Activity
Selective inhibitor of myosin II ATPase activity (IC 50 ~0.5-5 μ M); active enantiomer of (±)-blebbistatin ((?-1,2,3,3a-Tetrahydro-3a-hydroxy-6-methyl-1-phenyl-4H-pyrrolo[2,3-b]quinolin-4-one ). Inhibits contraction of the cleavage furrow without disrupting mitosis or contractile ring assembly. Rapidly and reversibly blocks cell blebbing, and disrupts directed cell migration and cytokinesis in vertebrate cells.
Biochem/physiol Actions
(-)-Blebbistatin is a cell cycle inhibitor; selective inhibitor of non-muscle myosin II.
storage
Store at +4°C
References
[1]. straight af, cheung a, limouze j, et al. dissecting temporal and spatial control of cytokinesis with a myosin ii inhibitor. science, 2003, 299:1743–1747.
[2]. kovacs m, toth j, hetenyi c, malnasi-csizmadia a, sellers jr. mechanism of blebbistatin inhibition of myosin ii. j biol chem, 2004, 279:35557–35563.
[3]. limouze j, straight af, mitchison t, sellers jr. specificity of blebbistatin, an inhibitor of myosin ii. j muscle res cell motil, 2004, 25:337–341.
[4]. miguel vicente-manzanares, xuefei ma, robert s. adelstein,alan rick horwitz. non-muscle myosin ii takes centre stage in cell adhesion and migration.nat rev mol cell biol, 2009 nov, 10(11): 778–790.
[5]. burt ra, joseph je, milliken s, collinge je, kile bt. description of a novel mutation leading to myh9-related disease. thrombosis research, 2008, 122(6): 861-863.
[6]. butcher dt, alliston t, weaver vm. a tense situation: forcing tumour progression. nature reviews cancer, 2009 feb, 9(2):108-122.
[7]. chen y, boukour s, milloud r, favier r, saposnik b, schlegel n, et al. the abnormal proplatelet formation in myh9-related macrothrombocytopenia results from an increased actomyosin contractility and is rescued by myosin iia inhibition. journal of thrombosis and haemostasis : jth , 2013, 11:2163-2175.
[8]. fedorov vv, lozinsky it, sosunov ea, anyukhovsky ep, rosen mr, balke cw, et al. application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. heart rhythm: the official journal of the heart rhythm society, 2007, 4:619-626.
[9]. zhang x-h, aydin m, kuppam d, melman a, disanto me. in vitro and in vivo relaxation of corpus cavernosum smooth muscle by the selective myosin ii inhibitor, blebbistatin. the journal of sexual medicine, 2009, 6:2661-2671.
[10]. zhang m, rao pv. blebbistatin, a novel inhibitor of myosin ii atpase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes. investigative ophthalmology & visual science, 2005, 46:4130-4138.
[11]. lucas-lopez c, allingham js, lebl t, lawson cp, brenk r, sellers jr, et al. the small molecule tool (s)-(-)-blebbistatin: novel insights of relevance to myosin inhibitor design. organic & biomolecular chemistry, 2008, 6:2076-2084.
Properties of (-)-Blebbistatin
| Melting point: | 210-212°C |
| Boiling point: | 507.3±60.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble5mg/mL |
| form | Yellow solid |
| pka | 11.15±0.20(Predicted) |
| color | Light Yellow to Yellow |
Safety information for (-)-Blebbistatin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (-)-Blebbistatin
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 (−)-Blebbistatin CAS 856925-71-8View Details
(−)-Blebbistatin CAS 856925-71-8View Details
856925-71-8 -
 (-)-Blebbistatin CAS 856925-71-8View Details
(-)-Blebbistatin CAS 856925-71-8View Details
856925-71-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1