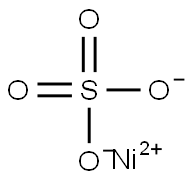
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Anhydrous nickel sulfate is a yellow-green crystalline solid. Nickel sulfate can also be obtained as a hexahydrate (NiSO4.6H2O (CAS: 10101-97-0) which is blue to emerald green, and as a heptahydrate (NiSO4.7H2O) (CAS: 10101-98-1) , which is green. Samples can contain variable quantities of water, depending on their previous exposure to moisture or conditions. All forms are mildly toxic and are carcinogenic. All are denser than water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used to make other nickel compounds, in printing, and in dyeing of textiles. |
|---|---|
| Color/Form | Green-yellow crystals |
| Odor | Odorless |
| Boiling Point | Decomposes (NTP, 1992) |
| Melting Point | 1558 °F (decomposes) (NTP, 1992) |
| Solubility | 27.3 to 27.7 % weight % at 68 °F (NTP, 1992) |
| Density | 3.68 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Stability/Shelf Life | STABLE @ 40 °C /HEXAHYDRATE/ |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | When heated to decomp it emits very toxic fumes of /sulfur oxides/. |
| Other Experimental Properties | Somewhat efflorescent; loses 5 H20 @ about 100 °C /Hexahydrate/ |
| Chemical Classes | Metals -> Nickel Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H334:Sensitisation, respiratory H351:Carcinogenicity H360:Reproductive toxicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 154.76 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 153.887071 g/mol |
| Monoisotopic Mass | 153.887071 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Anhydrous nickel sulfate is a yellow-green crystalline solid. Nickel sulfate can also be obtained as a hexahydrate (NiSO4.6H2O (CAS: 10101-97-0) which is blue to emerald green, and as a heptahydrate (NiSO4.7H2O) (CAS: 10101-98-1) , which is green. Samples can contain variable quantities of water, depending on their previous exposure to moisture or conditions. All forms are mildly toxic and are carcinogenic. All are denser than water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used to make other nickel compounds, in printing, and in dyeing of textiles.