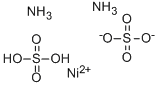CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Nickel ammonium sulfate is a green crystalline solid. Mildly toxic, carcinogenic. When heated to decomposition it emits highly toxic fumes of metallic nickel, oxides of sulfur, and oxides of nitrogen (Lewis, 3rd ed., 1993, p. 910). The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It is used for electroplating nickel. |
|---|---|
| Solubility | SOLUBILITY: 10.4 G/100 CC OF WATER AT 20 °C; 30 G/100 CC OF WATER AT 80 °C /HEXAHYDRATE/ |
| Density | 1.92 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Decomposition | When heated to decomp ... emits toxic fumes of /nitrogen oxides, sulfur oxides, and nickel/. |
| Refractive Index | Index of refraction: 1.495, 1.501, 1.508 /Hexahydrate/ |
| Other Experimental Properties | DARK BLUE-GREEN MONOCLINIC CRYSTALS /HEXAHYDRATE/ |
| Chemical Classes | Metals -> Nickel Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 286.90 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 285.907549 g/mol |
| Monoisotopic Mass | 285.907549 g/mol |
| Topological Polar Surface Area | 179 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 5 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Nickel ammonium sulfate is a green crystalline solid. Mildly toxic, carcinogenic. When heated to decomposition it emits highly toxic fumes of metallic nickel, oxides of sulfur, and oxides of nitrogen (Lewis, 3rd ed., 1993, p. 910). The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It is used for electroplating nickel.