10101-97-0
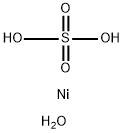
Product Name:
Nickel sulfate hexahydrate
Formula:
H4NiO5S
Synonyms:
Nickel(II) sulfate hexahydrate;Nickel(II) Sulfate, Hexahydrate, Nickel Sulfate;Nickelous Sulfate, Hexahydrate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Nickel sulfate hexahydrate appears as blue or emerald-green crystalline material. Toxic and carcinogenic. Two known phases. Alpha-form (blue tetragonal) converts to beta-form (green monoclinic) at 127.9 °F. Becomes blue and opaque at room temperature. Odorless. Sweet astringent taste. Somewhat efflorescent. Greenish-yellow anhydrous salt is formed at 536 °F. (NTP, 1992) |
|---|---|
| Boiling Point | Decomposes at 1544 °F (NTP, 1992) |
| Melting Point | 127.9 °F (transition point) (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 68 °F (NTP, 1992) |
| Density | 2.07 (NTP, 1992) - Denser than water; will sink |
| Vapor Pressure | 0 mmHg at 68 °F Essentially (NTP, 1992) |
| Chemical Classes | Metals -> Nickel Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H334:Sensitisation, respiratory H341:Germ cell mutagenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 262.85 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 0 |
| Exact Mass | 261.950459 g/mol |
| Monoisotopic Mass | 261.950459 g/mol |
| Topological Polar Surface Area | 94.6 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 8 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Nickel sulfate hexahydrate appears as blue or emerald-green crystalline material. Toxic and carcinogenic. Two known phases. Alpha-form (blue tetragonal) converts to beta-form (green monoclinic) at 127.9 °F. Becomes blue and opaque at room temperature. Odorless. Sweet astringent taste. Somewhat efflorescent. Greenish-yellow anhydrous salt is formed at 536 °F. (NTP, 1992)