Zoxazolamine
Synonym(s):Zoxazolamine
- CAS NO.:61-80-3
- Empirical Formula: C7H5ClN2O
- Molecular Weight: 168.58
- MDL number: MFCD00005770
- EINECS: 200-519-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
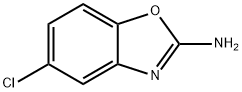
What is Zoxazolamine?
Chemical properties
LIGHT BROWN TO YELLOW-BROWN POWDER
Originator
Flexin ,McNeil,US,1956
The Uses of Zoxazolamine
Zoxazolamine is a centrally acting myorelaxant; formerly used as an antispasmodic and uricosuric. Used as a tool for assessing hepatic cytochrome P-450 activity in rodents.
The Uses of Zoxazolamine
sweetener
What are the applications of Application
Zoxazolamine is a centrally acting myorelaxant
Definition
ChEBI: Zoxazolamine is a benzoxazole.
Manufacturing Process
To a solution of 106 g (0.74 mol) of 2-amino-4-chlorophenol in 500 ml of
water containing 69 ml of concentrated hydrochloric acid (29.2 g, 0.8 mol) are
added 60.8 g (0.8 mol) of ammonium thiocyanate. The solution is placed in
an evaporating dish and heated on a steam bath for 5 hours. The solid which
results is then removed from the concentrated solution by filtration, washed
with a small amount of water and dried. The filtrate is placed in an
evaporating dish and heated on a water bath for 2 hours. At the end of this
time, the mixture is cooled, and the solid which precipitates out is removed by
filtration. Both solid products are 5-chloro-2-hydroxyphenylthiourea melting at
157°C, and may be combined. The calculated N content for C 7 H 7 ClN 2 OS is
13.8; that found is 13.6.
To a solution of 10 g (0.05 mol) of 2-hydroxy-5-chlorophenylthiourea in 50 ml
of methanol is added a solution of 11 g (0.04 mol) of ferric chloride
hexahydrate in 50 ml of methanol. The initial purple-red color changes in a
few minutes to amber. After stirring for one half hour, the solution is treated
with 16.5 ml of 57% ammonium hydroxide solution (0.24 mol). A brown,
flocculent precipitate of ferric sulfide appears. The mixture is then refluxed
with stirring for one hour, cooled and centrifuged. The centrifugate is
evaporated to dryness, and the residue is shaken with ether and water to
separate the organic material from the ammonium chloride. The ether layer is
extracted three times with 25 ml portions of 1 N hydrochloric acid. The acid
solution is then poured into excess ammonium hydroxide, and the resulting
solid collected, washed with water and dried. This gives a light tan solid
melting at 183°C to 185°. The material is then dissolved in 25 ml of acetone
and 50 ml of benzene are added. After treatment of the solution with
activated charcoal, the light yellow solution is evaporated to 25 ml and cooled.
The white crystals of 2-amino-5-chlorobenzoxazole which separate melt at
185°C to 186°C.
Therapeutic Function
Muscle relaxant, Uricosuric
Properties of Zoxazolamine
| Melting point: | 181-184 °C(lit.) |
| Boiling point: | 316.8±34.0 °C(Predicted) |
| Density | 1.4369 (rough estimate) |
| refractive index | 1.5618 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMSO, Methanol |
| form | Powder |
| pka | 0.73±0.10(Predicted) |
| color | Light brown to yellow-brown |
| Merck | 13,10249 |
| CAS DataBase Reference | 61-80-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Zoxazolamine(61-80-3) |
Safety information for Zoxazolamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zoxazolamine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 2-Amino-5-chlorobenzoxazole CAS 61-80-3View Details
2-Amino-5-chlorobenzoxazole CAS 61-80-3View Details
61-80-3 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4