Zirconium oxychloride
Synonym(s):Zirconium oxychloride;Zirconyl chloride;Dichlorooxozirconium;Oxozirconium(IV) chloride;Zirconium dichloride monoxide
- CAS NO.:7699-43-6
- Empirical Formula: Cl2OZr
- Molecular Weight: 178.13
- MDL number: MFCD00011309
- EINECS: 231-717-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
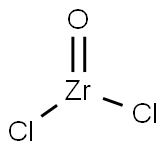
What is Zirconium oxychloride?
Chemical properties
Zirconium oxychloride exists as white silky crystals, soluble in water and alcohol. With water, the octahydrate is formed as tetragonal white needles. It is extremely hygroscopic and decomposes to zirconium tetrachloride (ZrCl4) and zirconium oxide (ZrO2) at 250 C. It is also formed as the octahydrate [13520-92-8] as white, tetrahedral crystals that are very soluble in water, ethyl alcohol, ether, and methanol, among others, but only slightly soluble in hydrochloric acid. When heated to decomposition, it emits toxic fumes of hydrogen chloride.
The Uses of Zirconium oxychloride
Textile, cosmetic and grease additive, antiperspirant, water repellents, chemical reagent, zirconium salts, in lakes and toners of acidic and basic dyes, oil-field acidizing aid.
The Uses of Zirconium oxychloride
Building block for a new series of mixed zirconium phosphonates which may have uses in catalysis, ion exchange or photophysics.
Definition
ChEBI: Zirconyl chloride is a zirconium coordination entity consisting of zirconium(IV) bound to oxygen via a double bond and to two chlorines.
Reactivity Profile
Acidic salts, such as Zirconium oxychloride, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
Has only a mild pharmacological action. Inhalation of dust may irritate nose and throat. Contact with eyes or skin causes irritation.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion and subcutaneous routes. Questionable carcinogen with experimental neoplastigenic data. When heated to decomposition it emits toxic fumes of Cl-. Used as an antiperspirant. See also ZIRCONIUM COMPOUNDS and CHLORIDES.
Purification Methods
Crystallise it repeatedly from 8M HCl to give ZrOCl2.8H2O (see below). On drying, ZrOCl2.6H2O, m 150o, is formed. The product is not free from hafnium. [Blumenthal J Chem Ed 39 607 1962.]
Properties of Zirconium oxychloride
| Melting point: | −15 °C |
| Density | 1.344 g/mL at 25 °C |
| solubility | soluble in H2O, ethanol |
| form | Liquid |
| color | Clear colorless |
| Merck | 13,10237 |
| Stability: | Stable. Incompatible with strong oxidizing agents, water, moisture, alkali metals. |
| CAS DataBase Reference | 7699-43-6(CAS DataBase Reference) |
| EPA Substance Registry System | Zirconium, dichlorooxo- (7699-43-6) |
Safety information for Zirconium oxychloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zirconium oxychloride
Zirconium oxychloride manufacturer
Bhalla Chemical Works Private Limited
New Products
Tetrabutylammonium iodide (3,3-DIFLUOROCYCLOBUTYL)METHANOL 4,4-DIFLUOROCYCLOHEXANAMINE Cyclobutylamine (S)-3-Fluoro-pyrrolidine hydrochloride 3-Oxocyclobutanecarboxylic acid N-Hydroxy-2-methylpropanimidamide L-tert-Leucine,97% 2-Bromophenylacetonitrile, 97% Aluminum oxide, basic Calcium hydroxide, 95% Diallylamine, 99% 2-Iodobenzoic Acid 3-Methoxybenzonitrile Pentachlorobenzonitrile Chloral Dibenzoyl Peroxide Titanium Dioxide O-Benzylhydroxylamine Hydrochloride 2-Nitrobenzaldehyde 2-Picolylamine (2-Aminomethylpyridine) 2-Venylpyridine Ethyl-2-Chloroacetoacetate 4-Dimethylamine PyridineRelated products of tetrahydrofuran
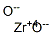

You may like
-
 Zirconyl chloride 98%View Details
Zirconyl chloride 98%View Details
7699-43-6 -
 Zirconyl chloride 98%View Details
Zirconyl chloride 98%View Details
7699-43-6 -
 7699-43-6 Zirconyl chloride 98%View Details
7699-43-6 Zirconyl chloride 98%View Details
7699-43-6 -
 1126-74-5 3-Pyridineacrylic acid 98+View Details
1126-74-5 3-Pyridineacrylic acid 98+View Details
1126-74-5 -
 tert-Butyl carbazate 98+View Details
tert-Butyl carbazate 98+View Details
870-46-2 -
 TETRABUTYLAMMONIUM CYANIDE 10442-39-4 98+View Details
TETRABUTYLAMMONIUM CYANIDE 10442-39-4 98+View Details
10442-39-4 -
 91419-52-2 98+View Details
91419-52-2 98+View Details
91419-52-2 -
 19752-84-2 TETRAHYDRO-2H-PYRAN-3-OL 98+View Details
19752-84-2 TETRAHYDRO-2H-PYRAN-3-OL 98+View Details
19752-84-2
