ZIRCONIUM HYDRIDE
Synonym(s):Zirconium dihydride
- CAS NO.:7704-99-6
- Empirical Formula: Zr
- Molecular Weight: 91.22
- MDL number: MFCD00148878
- EINECS: 231-727-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-30 15:22:23
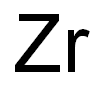
What is ZIRCONIUM HYDRIDE?
Description
Zirconium(II) hydride, ZrH2, is a molecular chemical compound, which has been produced by laser ablation and isolated at low temperature. Zirconium(II) hydride has a dihedral (C2v) structure. In zirconium(II) hydride, the formal oxidation states of hydrogen and zirconium are 21 and 12, respectively, since the electronegativity of zirconium is lower than that of hydrogen. The stability of metal hydrides with the formula MH2 (M 5 Ti-Hf) decreases as the atomic number increases.
Chemical properties
Gray-black metallic powder. Stable toward air and water.
Physical properties
Grayish-black powder; density 5.60 g/cm3; stable in water; soluble in dilute hydrofluoric acid; soluble in concentrated acids.
The Uses of ZIRCONIUM HYDRIDE
Zirconium hydride is a powerful reducing agent in acid solution or at high temperatures; hydrogenation catalyst; in the vacuum tube industry.Also, it is used as a source of pure hydrogen and a catalyst in hydrogenation reactions. Some other applications are in powder metallurgy; as a moderator in nuclear reactors; and as a metal-foaming agent
The Uses of ZIRCONIUM HYDRIDE
Zirconium Hydride, ZrH2, is a brittle, metallicgray solid that is stable in air and water, and has a density of 5.6 g/cm3. The chemical properties of ZrH2 closely resemble those of titanium hydride. Commercial uses are as a getter in the manufacture of vacuum tubes and other systems; as a hydrogen source for foaming metals; as a hydrogen reservoir; for the introduction of zirconium into powdered alloys; for metal–ceramic and metal–metal bonding; as a moderator in nuclear reactors; and as a source of Zr metal powder and alloys.
The Uses of ZIRCONIUM HYDRIDE
Zirconium(II) hydride (ZrH2) can be used as a precursor for:
- ?Reactive hot-pressing synthesis of Zr2SC ceramics by displacement reaction with the mixture of ZrC and ZrS2.,·?
- ?The synthesis of Mg-Zr hydrides by treating with MgH2 for hydrogen storage.???????
- The preparation of layer structured β-zirconium nitride chloride (ZrNCl) by reacting with NH4Cl.
Definition
Contains 1.7–2.1% combined hydrogen which can be driven off in a vacuum above 600C.
Preparation
Zirconium hydride may be prepared by heating zirconium oxide with magnesium in the presence of hydrogen: ZrO2 + 2Mg + H2 → ZrH2 + 2MgO
Alternatively, hydride may be made by heating zirconium oxide with calcium hydride in the presence of hydrogen
Hydride also may be obtained by combining zirconium metal with hydrogen at elevated temperature.
Production Methods
Zirconium hydride in powder form was produced by the reduction of zirconium oxide with calcium hydride in a bomb reactor. However, the workup was hazardous and many fires and explosions occurred when the calcium oxide was dissolved with hydrochloric acid to recover the hydride powder. With the ready availability of zirconium metal via the Kroll process, zirconium hydride can be obtained by exothermic absorption of hydrogen by pure zirconium, usually highly porous sponge. The heat of formation is 167.4 J/mol (40 kcal/mol) hydrogen absorbed.
General Description
This product has been enhanced for energy efficiency.
Hazard
Flammable, dangerous fire risk, especially in the presence of oxidizers.
Flammability and Explosibility
Flammable
Safety Profile
A powerful reducing agent. Flammable when dry or wet. Very dangerous to handle; can explode. Incandesces when heated in air. See also HYDRIDES and ZIRCONIUM COMPOUNDS.
Properties of ZIRCONIUM HYDRIDE
| Density | 5.47 |
| solubility | insoluble in H2O |
| form | Powder |
| color | gray tetragonal crystals, crystalline |
| Water Solubility | no reaction with H2O [MER06] |
| Sensitive | Moisture Sensitive |
| Merck | 14,10176 |
| CAS DataBase Reference | 7704-99-6(CAS DataBase Reference) |
| EPA Substance Registry System | Zirconium hydride (ZrH2) (7704-99-6) |
Safety information for ZIRCONIUM HYDRIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ZIRCONIUM HYDRIDE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Zirconium hydride, 99% CAS 7704-99-6View Details
Zirconium hydride, 99% CAS 7704-99-6View Details
7704-99-6 -
 Zirconium(II) hydride CAS 7704-99-6View Details
Zirconium(II) hydride CAS 7704-99-6View Details
7704-99-6 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4