Zinc fluoride
- CAS NO.:7783-49-5
- Empirical Formula: F2Zn
- Molecular Weight: 103.39
- MDL number: MFCD00011298
- EINECS: 232-001-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
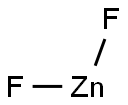
What is Zinc fluoride?
Description
Zinc fluoride (ZnF2) is an inorganic chemical compound. Zinc fluoride is used in the fluorination of organic compounds, in the manufacturing of phosphors for fluorescent lights, in preserving wood, in electroplating baths, in galvanizing steel, and in ceramic manufacturing for glazes and enamels. It is also used as a termite repellent, in pharmaceuticals (e.g. the inhibition of dentin demineralization and collagen degradation), as a flux for welding and soldering (e.g. aluminum).
Chemical properties
White powder. Soluble in hot acids; slightly soluble in water; insoluble in alcohol.
Physical properties
Anhydrous zinc fluoride is a white hygroscopic solid; tetragonal needles; density 4.9 g/cm3; melts at 872°C; vaporizes at 1,500°C; vapor pressure 1 torr at 1,243°C and 5 torr at 1,328°C; practically insoluble in water, 5.2 mg/L; sparingly soluble in HCl, HNO3 and ammonia solution
The hydrated salt, ZnF2?4H2O, is a white crystalline solid; rhombohedral crystals; density 2.30 g/cm3; loses water of crystallization at 100°C; sparinglysoluble in water, about 1.52 g/100mL at 20°C.
The Uses of Zinc fluoride
Zinc fluoride is a white crystalline powder, used in the manufacture of phosphors for fluorescent lights. It also is used in electroplating baths, in preservation of wood, in glazes and enamels for ceramics, and in fluorination reactions of organics.
The Uses of Zinc fluoride
Zinc Difluoride inhibits the erosion of enamel in vitro. It also functions as a fluorinating agent, Lewis acid catalyst and as an additive for catalytic transformations.
Preparation
Zinc fluoride may be prepared by heating zinc hydroxide or zinc carbonate with hydrogen fluoride:
Zn(OH)2 + 2HF → ZnF2 + 2H2O
ZnCO3 + 2HF → ZnF2 + CO2 + H2O
Also, it can be precipitated by adding a solution of sodium fluoride to that of zinc acetate:
(CH3COO)2Zn + 2NaF → ZnF2 + 2CH3COONa
Hazard
Toxic material.
Flammability and Explosibility
Not classified
Safety Profile
Poison by subcutaneous route. Can react violently with potassium. A fluorination agent. When heated to decomposition it emits toxic fumes of F and ZnO. See also FLUORIDES and ZINC COMPOUNDS.
Purification Methods
A possible impurity is H2O which can be removed by heating at 100o or by heating to 800o in a dry atmosphere. Heating in the presence of NH4F produces larger crystals. It is sparingly soluble in H2O (1.51g/100mL) but more soluble in HCl, HNO3 and NH4OH. It can be stored in glass bottles. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 242 1963.]
References
[1] http://www.npi.gov.au/resource/zinc-and-compounds
[2] https://hazmap.nlm.nih.gov/category-details?id=7782&table=copytblagents
[3] Patent EP 0001677 A1: Flux for soft soldering of aluminum, a fluxed solder composition containing said flux and a method of soft soldering aluminum using said flux
Properties of Zinc fluoride
| Melting point: | 872 °C (lit.) |
| Boiling point: | 1500 °C |
| Density | 4.95 g/mL at 25 °C (lit.) |
| vapor pressure | 1 mm Hg ( 970 °C) |
| Flash point: | 1500°C |
| solubility | Sparingly soluble in hydrochloric acid, nitric acid, and ammonium hydroxide. |
| form | powder |
| color | White to beige to light brown |
| Specific Gravity | 4.95 |
| Water Solubility | SLIGHTLY SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,10136 |
| Solubility Product Constant (Ksp) | pKsp: 1.52 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7783-49-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Zinc difluoride(7783-49-5) |
| EPA Substance Registry System | Zinc difluoride (7783-49-5) |
Safety information for Zinc fluoride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Zinc fluoride
Zinc fluoride manufacturer
Madras Fluorine Private Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Zinc fluoride 98%View Details
Zinc fluoride 98%View Details -
 Zinc fluoride CAS 7783-49-5View Details
Zinc fluoride CAS 7783-49-5View Details
7783-49-5 -
 Zinc fluoride CAS 7783-49-5View Details
Zinc fluoride CAS 7783-49-5View Details
7783-49-5 -
 Zinc fluoride CAS 7783-49-5View Details
Zinc fluoride CAS 7783-49-5View Details
7783-49-5 -
 Zinc fluoride CAS 7783-49-5View Details
Zinc fluoride CAS 7783-49-5View Details
7783-49-5 -
 Zinc fluoride CAS 7783-49-5View Details
Zinc fluoride CAS 7783-49-5View Details
7783-49-5 -
 Zinc fluoride CAS 7783-49-5View Details
Zinc fluoride CAS 7783-49-5View Details
7783-49-5 -
 Zinc Fluoride pure CAS 7783-49-5View Details
Zinc Fluoride pure CAS 7783-49-5View Details
7783-49-5