Zinc borate
- CAS NO.:1332-07-6
- Empirical Formula: B2O6Zn3
- Molecular Weight: 313.79
- MDL number: MFCD00069397
- EINECS: 215-566-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
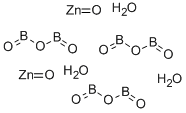
What is Zinc borate ?
Chemical properties
White, amorphous powder. Soluble in dilute acids; slightly soluble in water. Nonflammable.
Characteristics
Zinc Borate is a Boron based flame retardant compatible with many polymeric matrices. It is effective both in the solid phase and in the gas phase and its strong smoke suppressing action, helps to improve time of rescue in case of fire.
Zinc Borate is a multifunctional flame retardant:
promotes the formation of a protective vitreous layer and of a strong char layer, which reduces the formation of toxic and irritant smoke during the fire
It looses its water of hydration at temperatures above 290°C, cooling the front of the flames and subtracting energy to the fire
It acts as a synergist in conjunction with halogenated compounds, so that lower loadings of halogenated flame retardant additives are needed
It shows a strong synergic effect with antimony trioxide; in presence of alumina trihydrate (ATH) the synergic effect is enhanced
It improves resistance against electrical degradation: high anti-arcing and anti-tracking indexes
It is an afterglow suppressant.
The Uses of Zinc borate
Zinc borate is prepared as an insoluble double salt from water-soluble zinc and boron compounds. Compounds having varying amounts of zinc, boron, and water of hydration are available. The ratio of these components affects the temperature at which the flame-inhibiting powers are activated, as well as the temperature at which they can be processed. Zinc borates can either be used alone or in combination with other halogen synergists, such as antimony oxide. In some instances zinc borate is also used with alumina trihydrate to form a glass-like substance that inhibits polymer degradation.
The Uses of Zinc borate
Medicine, fireproofing textiles, fungistat and mildew inhibitor, flux in ceramics.
What are the applications of Application
zinc borate is primarily used as a flame retardant in plastics and cellulose fibers, paper, rubbers and textiles. It is also used in paints, adhesives, and pigments. As a flame retardant, it can replace antimony trioxide as a synergist in both halogen-based and halogen-free systems. It is an anti-dripping and char-promoting agent, and suppresses the afterglow. In electrical insulator plastics it suppresses arcing and tracking.
In halogen-containing systems zinc borate is used together with antimony trioxide and alumina trihydrate. It catalyzes formation of char and creates a protective layer of glass. Zinc catalyzes the release of halogens by forming zinc halides and zinc oxyhalides.
In halogen-free systems, zinc borate can be used together with alumina trihydrate, magnesium hydroxide, red phosphorus, or ammonium polyphosphate. When burning the plastics, a porous borate ceramic is formed that protects the underlying layers. In presence of silica, borosilicate glass can be formed at plastic burning temperatures.
As the partial, or completely EPA approved substitute for containing halogen and other flame retardants, zinc borate is being directly applied to a wide range of plastics and rubber processing such as PVC, PE, PP, and to enhance polyamide, PVC resin, polyphenylene ethylene, epoxy resin, polyester resin, acid ethylene and natural rubber, styrene butadiene rubber, and chloroprene rubber. It can also be applied to the production of paper, fiber fabric, decorative panels, floor leather, wallpaper, carpet, ceramic glaze, fungicides, and paint production to improve flame retardant performance.
Production Methods
Zinc borate (2ZnO·3B2O3·3.5H2O) in general is produced with the reaction between zinc oxide and boric acid. Boric acid is solved in water between temperatures 95ºC and 98ºC and zinc oxide and seed crystal of 2ZnO·3B2O3·3.5H2O is added to this solution at a certain stoichiometric ratio. The reaction continues for a while by mixing and the zinc borate formed is filtered, dried and ground. The boric acid solution is fed to the system as reflux.
Flammability and Explosibility
Not classified
Industrial uses
zinc borate (2ZnO-3B2Ovl5H2O) has a greater flame retardancy than borates used alone.
Zinc compounds perform most of their flame retardant function in the condensed phase. Zinc is used with boron in the form of zinc borate, and with molybdenum in the form of zinc molybdate. Zinc borate can also be used as a flame retardant and smoke suppressant with different polymers. In the case of zinc borate, 2ZnO.3B203.3.5H20, water given off can promote the formation of a cellular char which can act as a good insulator in protecting the underlying polymer or substrate. Moore studied the effects of zinc borate on smoke reduction and flame retardancy of PVC. He reported that smoke generation can be suppressed by over 40% by proper combination of additives without an adverse effect on flame retardancy. The use of zinc borate as a flame retardant and smoke suppressant alone or with other additives, e.g. Sb203, or AI(OH)3, has found wide application in the plastics industry.
Properties of Zinc borate
| Melting point: | 980 °C |
| Density | 3.64 g/cm3 |
| CAS DataBase Reference | 1332-07-6(CAS DataBase Reference) |
| EPA Substance Registry System | Zinc borate (1332-07-6) |
Safety information for Zinc borate
Computed Descriptors for Zinc borate
Zinc borate manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
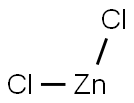
You may like
-
 Zinc borate 99%View Details
Zinc borate 99%View Details -
 Zinc borate 99%View Details
Zinc borate 99%View Details
1332-07-6 / 12536-65-1 -
 Zinc borate 1332-07-6 / 12536-65-1 98%View Details
Zinc borate 1332-07-6 / 12536-65-1 98%View Details
1332-07-6 / 12536-65-1 -
 1332-07-6 / 12536-65-1 Zinc borate 99%View Details
1332-07-6 / 12536-65-1 Zinc borate 99%View Details
1332-07-6 / 12536-65-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
