Zidapamide
- CAS NO.:75820-08-5
- Empirical Formula: C16H16ClN3O3S
- Molecular Weight: 365.83
- MDL number: MFCD00867337
- EINECS: 278-321-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
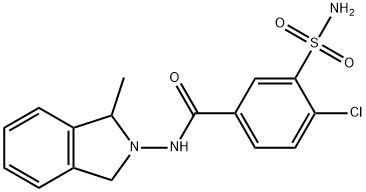
What is Zidapamide?
Originator
Zidapamide,ZYF Pharm Chemical
The Uses of Zidapamide
Zidapamide is a thiazide-like diuretic drug used in treatment of hypertension.
Manufacturing Process
5.6 ml of triethylamine are added to a solution of 5.0 g (0.018 m) of α-
methyl-α,α-dibromo-o-xylene and 2.38 g (0.018 m) of t-butylcarbazate in 15
ml of dimethylformamide heated to 50-60°C. After the addition, the mixture is
left stirring for 3 h at room temperature, the solution volume is then made up
to about 60 ml by diluting with H 2 O and the solution is left for a further hour
under stirring. The solid which separates is filtered off, washed with water and
dried to give 3.14 g (70%) of 1-methyl-N-(t-butyloxycarbonylamino)
isoindoline, melting point 143-145°C.
A suspension of 2.6 g (0.0104 m) of the 1-methyl-N-(t-
butyloxycarbonylamino)isoindoline in 7 ml of concentrated HCl is kept stirring
at room temperature for 1 h. The final solution is evaporated to dryness under
vacuum by heating to 60-70°C to give a solid residue (1.97 g) which
crystalises from EtOH+Et 2 O (1/1) to give 1.5 g (77.6%) of 1-methyl-2-amine
isoindoline hydrochloride, melting point 140-145°C.
3.44 g (0.0 135 m) of 4-chloro-3-sulfamidobenzoic acid chloride are added to
a solution of 2.5 g (0.0135 m) of 1-methyl-2-aminoisoindoline hydrochloride
and 3.5 g (0.0314 m) of triethylamine in 30 ml of tetrahydrofuran. The
mixture is kept stirring at room temperature for 15 h. The abundant solid
which separates is filtered off, and is suspended in water in order to remove
the triethylamine hydrochloride present. The residue is collected by filtration
and dried in a drier to give 3.0 g of 1-methyl-2-(3'-sulfamyl-4'-
chlorobenzamido)isoindoline, melting point 208-210°C (the analytical sample
crystallizes from 10 volumes of ethyl alcohol, and has a melting point of 210-
212°C).
A further amount (0.4 g) of product can be isolated by evaporating the
tetrahydrofuran reaction solution, mixing the oily residue with ethyl alcohol
and allowing it to crystallize in a refrigerator. The overall yield is 3.4 g, equal
to 68.6%.
Therapeutic Function
Antihypertensive
Safety information for Zidapamide
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran






You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4