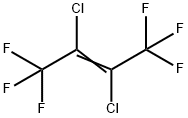
(Z)-1,1,1,4,4,4-HEXAFLUORO-2-BUTENE
- CAS NO.:692-49-9
- Empirical Formula: C4H2F6
- Molecular Weight: 164.05
- MDL number: MFCD10565642
- EINECS: 700-651-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:18:17
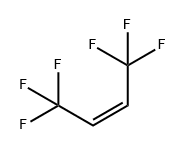
What is (Z)-1,1,1,4,4,4-HEXAFLUORO-2-BUTENE?
Description
Hexafluoro-2-butene, more specifically (Z)-1,1,1,4,4,4-hexafluoro-2-butene, is an unsaturated, highly fluorinated hydrocarbon with multiple industrial uses. It also goes by the rather bizarre commercial name 1336mzz(Z).
Hexafluoro-2-butene is used as a working fluid for organic Rankine power cycles, as a refrigerant for use in chillers, and as a foam-blowing agent. (A Rankine cycle is a construct used to evaluate the performance of steam turbine systems; “organic” refers to the use of an organic liquid with a lower boiling point than water in such a system.)
Despite the harmful properties shown in the hazard information table, the U.S. Environmental Protection Agency approved its industrial uses in 2017 under the agency’s Significant New Alternatives Policy. The driving force for this approval was hexafluoro-2-butene’s short atmospheric lifetime of 22 days that contrasts with ≈8 years for hydrofluorocarbon HFC-245fa, which has similar uses. The compound’s labile carbon–carbon double bond is the key to its short lifetime.
Another advantage of hexafluoro-2-butene is its very low 100-year global warming potential of 2 (carbon dioxide = 1) compared with 858 for HFC-245fa. It is relatively nontoxic and not flammable.
Although hexafluoro-2-butene was synthesized as early as 1952 by Robert N. Haszeldine at the University of Cambridge (UK), few of its thermodynamic and other physical properties have been published. Knowledge of hexafluoro-2-butene’s properties is important for manufacturers and users of industrial equipment, so Mark O. McLinden* at the National Institute of Standards and Technology (Boulder, CO) and Ryo Akasaka at Kyushu University (Fukuoka, Japan) undertook a comprehensive study of its properties.
The authors measured experimental data for the vapor pressure (including dew points), density, and vapor-phase sound speeds for hexafluoro-2-butene. Using these and previously reported data, they developed an equation of state based on Helmholtz free energy for the compound. The equation is valid for the temperature range 200–500 K and pressures ≤46 MPa.
The Uses of (Z)-1,1,1,4,4,4-HEXAFLUORO-2-BUTENE
Z-1,1,1,4,4,4-Hexafluoro-2-butene is a useful compound in regards to heat transfer applications due to the stability of the carbon fluorine bond.
Properties of (Z)-1,1,1,4,4,4-HEXAFLUORO-2-BUTENE
| Boiling point: | 9℃ |
| Density | 1.356 |
| vapor pressure | 60.435kPa at 20.01℃ |
| Flash point: | -21℃ |
| storage temp. | Refrigerator |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| form | Oil |
| appearance | colorless liquid |
| color | Colourless |
| Stability: | Very Volatile |
| EPA Substance Registry System | 2-Butene, 1,1,1,4,4,4-hexafluoro-, (2Z)- (692-49-9) |
Safety information for (Z)-1,1,1,4,4,4-HEXAFLUORO-2-BUTENE
Computed Descriptors for (Z)-1,1,1,4,4,4-HEXAFLUORO-2-BUTENE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

![2,3-BIS-TRIFLUOROMETHYL-THIENO[3,4-B][1,4]DIOXINE-5,7-DICARBOXYLIC ACID](https://img.chemicalbook.in/StructureFile/ChemBookStructure7/GIF/CB4391443.gif)

You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
