Hexafluoropropylene
Synonym(s):Hexafluoropropylene;Perfluoropropene
- CAS NO.:116-15-4
- Empirical Formula: C3F6
- Molecular Weight: 150.02
- MDL number: MFCD00000447
- EINECS: 204-127-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
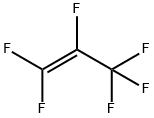
What is Hexafluoropropylene?
Chemical properties
Hexafluoropropylene (HFP) is a colourless, odourless, non-flammable gas that is only slightly soluble in water. It is used as chemical intermediate primarily in the synthesis of fluoropolymers, fluoro-elastomers and fluorinated materials (e.g. perfluoropolyether functional fluids, oils and greases).
The Uses of Hexafluoropropylene
Hexafluoropropene is used in the synthesis of polyvinylidene fluoride-co-hexafluoropropylene, a polymer which is currently being used in various fields of the nano and electronic sector ranging from membranes to nanoparticles.
Preparation
Hexafluoropropylene can be produced by pyrolysis of tetrafluoroethylene (TFE):
3 CF2=CF2 → 2 CF3CF=CF
This reaction is carried out in a closed continuous reactor at 600 to 900°C. The yield can be improved at low partial pressure, achieved either by operating under reduced total pressure (down to 0.1 bar) or by injecting an inert diluent such as steam or CO2.
Significant quantities of by-products, including the highly toxic perfluoroisobutylene (PFIB), are formed during the pyrolysis of TFE. Such by-products are either incinerated directly or are collected and transported for incineration at a remote facility.
Hexafluoropropylene can also be prepared from chlorodifluoromethane, or produced from various chlorofluorocarbons.
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 4054, 1951 DOI: 10.1021/ja01152a548
General Description
Hexafluoropropylene is an odorless, colorless gas. Hexafluoropropylene is noncombustible. Hexafluoropropylene can asphyxiate by the displacement of air. Exposure of the container to prolonged heat or fire can cause Hexafluoropropylene to rupture violently and rocket.
Reactivity Profile
Halogenated aliphatic compounds, such as Hexafluoropropylene, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Above a minimum oxygen pressure, the reaction of oxygen difluoride and hexafluoropropene to yield the Hexafluoropropylene oxide becomes explosive, Chem. Abs., 1987, 107, 175302. The reaction of hexafluoropropene with grignard reagent (subst. phenylmagnesium bromides) led to explosion, Fluorine Chem., 1981, 18, 25.
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket.
Flammability and Explosibility
Non flammable
Properties of Hexafluoropropylene
| Melting point: | −153 °C(lit.) |
| Boiling point: | −28 °C(lit.) |
| Density | 1,583 g/cm3 |
| vapor pressure | 587.952kPa at 25℃ |
| refractive index | 1.4000 |
| form | gas |
| Water Solubility | 82mg/L at 28℃ |
| BRN | 1705671 |
| Stability: | Stable. Incompatible with alkali metals, powdered metals. |
| CAS DataBase Reference | 116-15-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Propene, hexafluoro-(116-15-4) |
| EPA Substance Registry System | Hexafluoropropene (116-15-4) |
Safety information for Hexafluoropropylene
| Signal word | Warning |
| Pictogram(s) |
 Gas Cylinder Compressed Gases GHS04  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H280:Gases under pressure H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H371:Specific target organ toxicity, single exposure H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for Hexafluoropropylene
| InChIKey | HCDGVLDPFQMKDK-UHFFFAOYSA-N |
New Products
(R)-1-Boc-3-hydroxypyrrolidine Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 Hexafluoropropene, ≥99% CAS 116-15-4View Details
Hexafluoropropene, ≥99% CAS 116-15-4View Details
116-15-4 -
 Hexafluoropropene 99% CAS 116-15-4View Details
Hexafluoropropene 99% CAS 116-15-4View Details
116-15-4 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
