Xaliproden hydrochloride
Synonym(s):1,2,3,6-Tetrahydro-1-[2-(2-naphthalenyl)ethyl]-4-[3-(trifluoromethyl)phenyl]pyridine hydrochloride;SR-57746A
- CAS NO.:90494-79-4
- Empirical Formula: C24H23ClF3N
- Molecular Weight: 417.9
- MDL number: MFCD00896209
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 17:01:59
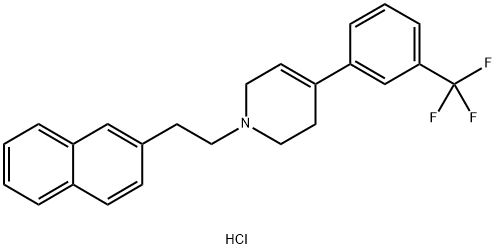
What is Xaliproden hydrochloride?
Originator
Xaliproden hydrochloride,Sanofi (Sanofi- Aventis)
The Uses of Xaliproden hydrochloride
Xaliproden is a 5-HT1A agonist used in the treatment of allodynia.
What are the applications of Application
Xaliproden hydrochloride is a full agonist of SR-1A
Definition
ChEBI: Xaliproden hydrochloride is a member of naphthalenes.
Manufacturing Process
A solution of 27.8 kg of 2-naphthylacetic acid in 95 L of tetrahydrofuran is
added, at a temperature below 20°C to a mixture of 27.5 L of tetrahydrofuran
and 10.0 kg of lithium aluminum hydride. The mixture is cooled to 0°C and
the following are then added slowly: firstly 10 L of water, then a solution of
1.5 kg of sodium hydroxide in 10 L of water, and finally 30 L of water. The
salts which separate out are washed with 160 L of tetrahydrofuran and then filtered off. The combined tetrahydrofuran solutions are evaporated and the
24.5 kg of 2-(2-naphthyl)ethanol are obtained.
The 2-(2-naphthyl)ethanol is treated with 138 L of concentrated hydrobromic
acid. The mixture is refluxed for 5 h and allowed to return to room
temperature, with stirring, and the product obtained is then filtered off and
washed with water. The moist product is dissolved in 147 L of isopropanol
under reflux, about 75 L of solvent are removed by distillation and the
mixture is allowed to cool overnight. The product which has crystallized is
filtered off, washed with previously cooled isopropanol and dried under
vacuum at 40°C. So the 2-(2-bromoethyl)naphthalene is obtained (yield:
81%, calculated relative to the starting naphthylacetic acid).
A mixture of 12.5 g of 2-(2-bromoethyl)naphthalene, 14.0 g of 4-(3-
trifluoromethylphenyl)-1,2,3,6-tetrahydropyridine hydrochloride, 4.34 g of
sodium hydroxide, 135 ml of water and 95 ml of 95% ethanol is refluxed for 5
h, the reaction mixture is subsequently allowed to cool to room temperature
overnight and then filtered and the product isolated in this way is washed with
water and dried under vacuum at 50°C to give 1-[2-(2-naphthyl)ethyl]-4-(3-
trifluoromethylphenyl)-1,2,3,6-tetrahydropyridine (yield of 90%, calculated
relative to the starting 4-(3-trifluoromethylphenyl)-1,2,3,6-tetrahydropyridine
hydrochloride).
Therapeutic Function
Serotonin antagonist, Nootropic
Biological Activity
Orally active, full agonist at 5-HT 1A receptors. Binds rat 5-HT 1A with high affinity (K i = 2.0 nM) and is > 300-fold selective over other 5-HT receptor subtypes (IC 50 > 650 nM). Increases motoneuron survival and promotes effects of NGF on neurite outgrowth in vitro . Displays neurotrophic activity in several neurodegenerative models in vivo .
Properties of Xaliproden hydrochloride
| Melting point: | 255-260° |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥5mg/mL |
| form | solid |
| color | white to off-white |
Safety information for Xaliproden hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Xaliproden hydrochloride
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



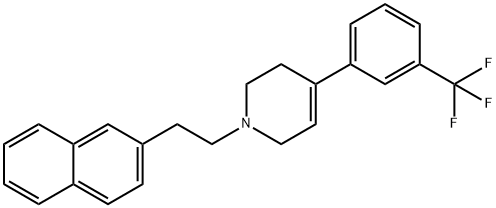

You may like
-
 Xaliproden hydrochloride CAS 90494-79-4View Details
Xaliproden hydrochloride CAS 90494-79-4View Details
90494-79-4 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4