viquidil
- CAS NO.:84-55-9
- Empirical Formula: C20H24N2O2
- Molecular Weight: 324.42
- MDL number: MFCD24682725
- EINECS: 201-540-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
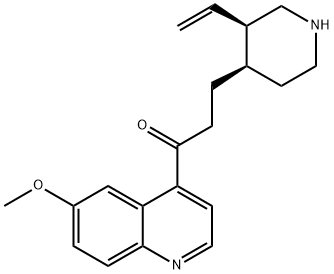
What is viquidil?
Originator
Desclidium,Spret ,France,1972
The Uses of viquidil
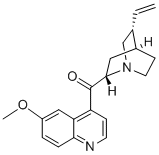
1-(6-Methoxyquinolin-4-yl)-3-((3R,4R)-3-vinylpiperidin-4-yl)propan-1-one (CAS# 84-55-9) is in intermediate in the synthesis of Quinotoxine Hydrochloride (Q753500), an isomer of quinine; occurs naturally as the d-form. Present in small quantities in cinchona barks. Vasodilator (cerebral).
Definition
ChEBI: Viquidil is a member of quinolines.
Manufacturing Process
2.70 g of N-benzoylhomomeroquinene ethyl ester (0.0086 mol) are mixed
with 4.0 g of ethyl quininate (0.0173 mol = 100% excess). 1.4 g of absolutely
dry pulverulent sodium ethoxide (0.0207 mol -140% excess, based on N-
benzoylhomomeroquinene ethyl ester) is added, and the reaction mixture is
heated to about 80°C with continuous stirring. As the ethyl quininate melts,
and the materials become thoroughly mixed, the initial yellow color changes
to brown and then gradually to deep red. The reaction mixture is maintained
at about 82°C for fourteen hours with continuous stirring. It is then cooled,
and the resulting very hard, dark red mass is decomposed with ice water and
benzene. The (not entirely clear) combined aqueous layers are extracted with
a small amount of ether. The clear, deep red, aqueous layer is then made just
acid to litmus. The precipitated oil is taken up in ether. Evaporation of solvent,
finally in vacuo, gives 2.56 g of a red glass. The combined benzene and ether
extracts from above, containing largely neutral material, are extracted with
10% aqueous sodium hydroxide. The alkaline extract is made just acid to
litmus, and extraction with ether followed by removal of solvent gives a
further small quantity of β-ketoester, 0.16 g.
Total weight of N-benzoylquinotoxine carboxylic acid ethyl ester thus obtained
was 2.72 g, equivalent to 63.4% of the theoretical.
2.72 g of N-benzoylquinotoxine carboxylic acid ethyl ester are dissolved in 30
cc of 1:1 aqueous hydrochloric acid (from 15 cc concentrated hydrochloric acid
and 15 cc water). The clear, reddish-orange solution is then boiled under
reflux for four hours. The very dark reddish-brown solution is extracted with
ether (from this extract 0.50 g of benzoic acid is obtained on evaporation).
The aqueous solution is then made strongly alkaline and extracted with ether.
0.23 g of ether-insoluble interface material is dissolved in benzene and set
aside. Removal of solvent from the above ether extract gives 1.39 g of crude
quinotoxine as a dark red viscous oil.
Therapeutic Function
Vasodilator, Antiarrhythmic
Properties of viquidil
| Melting point: | 60°C |
| Boiling point: | 462.75°C (rough estimate) |
| alpha | D +43° |
| Density | 1.1294 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| form | Solid |
| pka | 10.13±0.10(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for viquidil
Computed Descriptors for viquidil
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![6-[[3-[4-(2-Methoxyphenyl)-1-piperazinyl]propyl]amino]-1,3-dimethyluracil](https://img.chemicalbook.in/CAS/GIF/34661-75-1.gif)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1