Diltiazem
- CAS NO.:42399-41-7
- Empirical Formula: C22H26N2O4S
- Molecular Weight: 414.52
- MDL number: MFCD00868239
- EINECS: 255-796-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-12 11:51:25
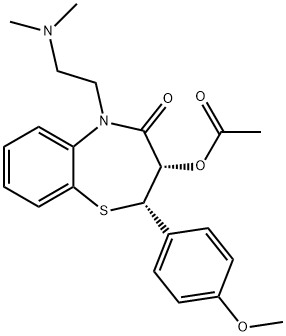
What is Diltiazem?
Absorption
Diltiazem is readily absorbed from the gastrointestinal tract. Minimum therapeutic plasma diltiazem concentrations appear to be in the range of 50 to 200 ng/mL. Following oral administration of extended formulations of 360 mg diltiazem, the drug in plasma was detectable within 3 to 4 hours and the peak plasma concentrations were reached between 11 and 18 hours post-dose. Diltiazem peak and systemic exposures were not affected by concurrent food intake. Due to hepatic first-pass metabolism, the absolute bioavailability following oral administration is about 40%, with the value ranging from 24 to 74% due to high interindividual variation in the first pass effect. The bioavailability may increase in patients with hepatic impairment.
Toxicity
Clinical Toxicity and Overdose
The oral LD50 ranges from 415 to 740mg/kg in mice and 560 to 810 mg/kg in rats. The oral LD50 in dogs is considered to be in excess of 50 mg/kg. A dose of 360 mg/kg resulted in lethality in monkeys. The intravenous LD50 is 60 mg/kg in mice and 38 mg/kg in rats.
Cases of overdose from doses ranging from less than 1 g to 18 g have been reported with diltiazem, with several cases involving multiple drug ingestions resulting in death. Overdoses were associated with bradycardia, hypotension, heart block, and cardiac failure that may manifest as dizziness, lightheadedness, and fatigue. Actual treatment and dosage should depend on the severity of the clinical situation and the judgment and experience of the treating physician. Diltiazem overdose should be responded with appropriate supportive measures and gastrointestinal decontamination. Bradycardia and heart block can be treated with atropine at doses ranging from 0.60 to 1.0 mg. In the case of bradycardia, if there is no response to vagal blockage, cautious administration of isoproterenol should be considered. Cardiac pacing can also be used to treat fixed high-degree AV block. In the case of heart failure, blood pressure may be maintained with the use of fluids and vasopressors, as well as inotropic agents such as isoproterenol, dopamine, or dobutamine. Other appropriate measures include ventilatory support, gastric lavage, activated charcoal, and/or intravenous calcium. Diltiazem does not appear to be removed by peritoneal or hemodialysis.
Non-clinical toxicity
In a 24-month study in rats receiving oral doses of up to 100 mg/kg/day, there was no evidence of carcinogenicity. There was also no mutagenic response in vitro or in vivo in mammalian cell assays or in vitro bacterial assays. No evidence of impaired fertility was observed in a study performed in male and female rats receiving oral doses of up to 100 mg/kg/day.
Pregnancy and Lactation
In reproduction studies in animals, administration of diltiazem at doses ranging from five to twenty times the daily recommended human therapeutic dose resulted in cases of the embryo and fetal lethality and skeletal abnormalities, and an increase in the risk of stillbirths. There have been no up-to-date controlled studies that investigated the use of diltiazem in pregnant women. The use of diltiazem in pregnant women should be undertaken only if the potential benefit justifies the risk to the fetus. Diltiazem is excreted in human milk, where one report suggests that the concentrations in breast milk may approximate serum levels; therefore, the decision should be made to either discontinue nursing or the use of the drug after careful consideration of the clinical necessity of diltiazem therapy in the nursing mother.
Use in special populations
As there is limited information on the variable effects of diltiazem in geriatric patients, the initial therapy of diltiazem should involve the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Currently, there are no specific dosing guidelines for patients with renal or hepatic impairment.
Originator
Herbesser,TANABE SEIYAKU,Japan,1974
The Uses of Diltiazem
Diltiazem is a potent vasodilator compound. Antihypertensive.
The Uses of Diltiazem
It is used for stable and nonstable angina pectoris (including after myocardial infarctions) as well as in arterial hypertension.
What are the applications of Application
Diltiazem is a potent vasodilator compound
Indications
Oral
Indicated for the management of hypertension, to lower blood pressure, alone or in combination with other antihypertensive agents.
Indicated for use to improve exercise tolerance in patients with chronic stable angina.
Indicated for the management of variant angina (Prinzmetal's angina).
Intravenous
Indicated for the short-term management of atrial fibrillation or atrial flutter for temporary control of rapid ventricular rate.
Indicated for the rapid conversion of paroxysmal supraventricular tachycardias (PSVT) to sinus rhythm. This includes AV nodal reentrant tachycardias and reciprocating tachycardias associated with an extranodal accessory pathway such as the WPW syndrome or short PR syndrome.
Off-label
Indicated for off-label uses in anal fissures (as topical formulation), migraine prophylaxis, cramps in lower leg related to rest, pulmonary hypertension, idiopathic dilated cardiomyopathy, and proteinuria associated with diabetic nephropathy.
Background
Diltiazem is a benzothiazepine derivative with antihypertensive and vasodilating properties. Approved in 1982 by the FDA, it is a member of the non-dihydropyridine calcium channel blockers drug class. It works through various mechanisms of action, but it primarily works by inhibiting the calcium influx into cardiac and vascular smooth muscle during depolarization. Compared to dihydropyridine drugs, such as nifedipine, that preferentially act on vascular smooth muscle and verapamil that directly acts on the heart muscle, diltiazem displays an intermediate specificity to target both the cardiac and vascular smooth muscle. Being a potent vasodilator, diltiazem is used clinically as an antihypertensive, anti-arrhythmic, and as an anti-anginal agent for the management of cardiovascular conditions such as hypertension, chronic stable angina, atrial fibrillation, atrial flutter. Apart from its main FDA-approved indications, diltiazem has also been used for numerous off-label indications, such as anal fissures (in topical formulations), migraine prophylaxis, pulmonary hypertension, and rest-related cramps in the lower extremities. Typically available in extended-release oral and intravenous formulations, diltiazem is marketed under various brand names with Cardizem and Tiazac being the most common ones.
Definition
ChEBI: A 5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl acetate in which both stereocentres have S configuration. A calcium-channel blocker and vasodilator, it is used as the hydrochloride in the m nagement of angina pectoris and hypertension.
Manufacturing Process
β-Diethylaminoethyl chloride is condensed with 2-(4-methoxyphenyl)-3-
hydroxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one in a first step. Then a
mixture of 1.5 grams of 2-(4-methoxyphenyl)-3-hydroxy-5-(βdimethylaminoethyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one and 20 ml of
acetic anhydride was heated on a water bath for 5 hours. The reaction
mixture was evaporated under reduced pressure to remove acetic anhydride
and the concentrated product was poured into ice water. The resulting mixture
was made alkaline with sodium bicarbonate and extracted with chloroform.
The chloroform layer was dried and evaporated to remove the solvent. The
residue was dissolved in acetone, and an ethanol solution containing hydrogen
chloride was added thereto producing 1.53 grams of 2-(4-methoxyphenyl)3-
acetoxy-5-(β-dimethylaminoethyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one
hydrochloride having a melting point from 187° to 188°C.
The starting material is made by reacting 4-methoxybenzaldehyde with ethyl
chloroacetate; that product with sodium ethoxide; and that product with 2-
aminothiophenol.
Therapeutic Function
Coronary vasodilator
Mechanism of action
Diltiazem reduces the transmembrane influx of calcium ions into cells of cardiac muscle and smooth musculature of vessels. It causes dilation of coronary and peripheral vessels, increases coronary blood flow, and prevents development of coronary artery spasms. It lowers elevated arterial pressure and reduces tachycardia.
Pharmacokinetics
Diltiazem is an antihypertensive and vasodilating agent that works by relaxing the vascular muscle and reducing blood pressure. This is related to the long-term therapeutic effects, as lowering the blood pressure reduces the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. Diltiazem inhibits the influx of extracellular calcium ions across the myocardial and vascular smooth muscle cell membranes during depolarization. Diltiazem is classified as a negative inotrope (decreased force) and negative chronotrope (decreased rate). It is also considered a rate-control drug as it reduces heart rate. Diltiazem is exerts hemodynamic actions by reducing blood pressure, systemic vascular resistance, the rate-pressure product, and coronary vascular resistance while increasing coronary blood flow. Diltiazem decreases sinoatrial and atrioventricular conduction in isolated tissues and has a negative inotropic effect in isolated preparations. In supraventricular tachycardia, diltiazem prolongs AV nodal refractories.
As the magnitude of blood pressure reduction is related to the degree of hypertension, the antihypertensive effect of diltiazem is most pronounced in individuals with hypertension. In a randomized, double-blind, parallel-group, dose-response study involving patients with essential hypertension, there was a reduction in the diastolic blood pressure by 1.9, 5.4, 6.1, and 8.6 mmHg in the patients receiving diltiazem at doses of 120, 240, 360, and 540 mg, respectively. In patients receiving placebo, there was a reduction in the diastolic blood pressure by 2.6 mmHg.In a randomized, double-blind study involving patients with chronic stable angina, variable doses of diltiazem administered at night all caused an increased exercise tolerance in the after 21 hours, compared to placebo. In the NORDIL study of patients with hypertension, the therapeutic effectiveness of diltiazem in reducing cardiovascular morbidity and mortality was assessed. When using the combined primary endpoint as fatal and non-fatal stroke, myocardial infarction, and other cardiovascular death, fatal and non-fatal stroke was shown to be reduced by 25% in the diltiazem group. Although the clinical significance to this effect remains unclear, it is suggested that diltiazem may exert a protective role against cerebral stroke in hypertensive patients.
Clinical Use
The antiarrhythmic actions and uses of diltiazem are similar to those of verapamil. Diltiazem is effective in controlling the ventricular rate in patients with atrial flutter or atrial fibrillation.
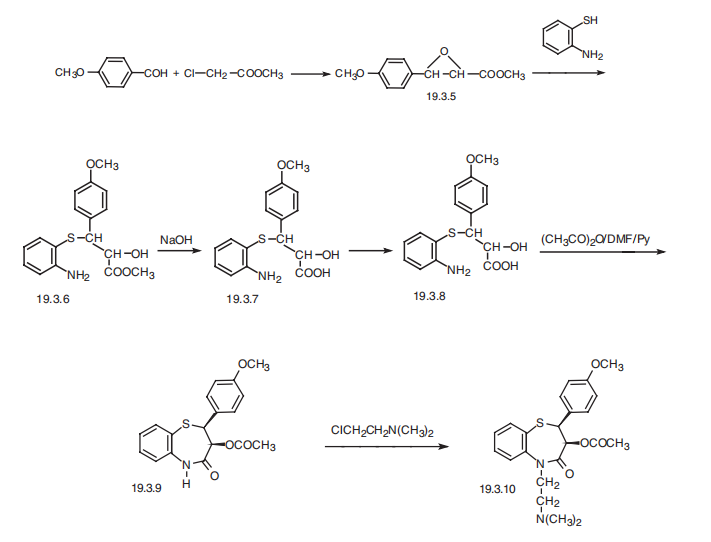
Synthesis
Diltiazem, 5-[2-(diethylamino)ethyl]-cis-2,3-dihydro-3-hydroxy-2- (4-methoxy-phenyl)-1,5-benzothiazepin-4(5H)-one (19.3.10), is synthesized in the following manner. The condensation of 4-methoxybenzaldehyde with methylchoroacetate in the presence of sodium methoxide in Darzens reaction conditions gives methyl ester of 3- (4-methoxyphenyl)-glycidylic acid (19.3.5). Reacting it with 2-aminothiophenol with the opening of epoxide ring gives methyl ester of 2-hydroxy-3-(2'-aminophenylthio)-3- (4"- methoxyphenyl)propionic acid (19.3.6). Hydrolysis of the resulting compound with alkali leads to the formation of the corresponding acid (19.3.7) in the form of a racemic mixture, which when on interaction with (+)-|á-phenylethylamine gives threo-(+)-2-hydroxy-3-(2'- aminophenylthio)-3-(4"-methoxyphenylpropionic acid (19.3.8). Boiling this in a mixture of acetic anhydride/dimethylformamide/pyridine system brings to cyclization to the thiazepine ring and simultaneously acylates the hydroxyl group, forming (+)-cis-2-(4-methoxyphenyl)- 3-acetoxy-2,3-dihydro-1,5-benzothiazepin-4-(5H)-one (19.3.9). Alkylation of the resulting product with 2,2-dimethylaminoethylchloride forms diltiazem (19.3.10).
Metabolism
Diltiazem is subject to extensive first-pass metabolism, which explains its relatively low absolute oral bioavailability. It undergoes N-demethylation primarily mediated by CYP3A4. CYP2D6 is responsible for O-demethylation and esterases mediate deacetylation. There was large inter-individual variability in the circulating plasma levels of metabolites in healthy volunteers.
In healthy volunteers, the major circulating metabolites in the plasma are N-monodesmethyl diltilazem, deacetyl diltiazem, and deacetyl N-monodesmethyl diltiazem, which are all pharmacologically active. Deacetyl diltiazem retains about 25-50% of the pharmacological activity to that of the parent compound. Deacetyl diltiazem can be further transformed into deacetyl diltiazem N-oxide or deacetyl O-desmethyl diltiazem. N-monodesmethyl diltilazem can be further metabolized to N,O-didesmethyl diltiazem. Deacetyl N-monodesmethyl diltiazem can be further metabolized to deacetyl N,O-didesmethyl diltiazem, which can be glucuronidated or sulphated. Diltiazem can be O-demethylated by CYP2D6 to form O-desmethyl diltiazem.
Properties of Diltiazem
| Melting point: | 212 °C (decomp) |
| Boiling point: | 594.4±50.0 °C(Predicted) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 7.70 (Uncertain) |
| color | White to Off-White |
| CAS DataBase Reference | 42399-41-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Diltiazem(42399-41-7) |
| EPA Substance Registry System | 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, (2S,3S)- (42399-41-7) |
Safety information for Diltiazem
Computed Descriptors for Diltiazem
Diltiazem manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
![42399-41-7 (2S-Cis)-3-(Acetyloxy)-5-[2-dimethylamino)ethyl]-2,3-dihydro-2-(4methoxy phenyl)- 1,5benzothiazepin-4(5H)-one D 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/2f88eb5d-bb38-4ff7-88c3-a8b5cccf96d1.png) 42399-41-7 (2S-Cis)-3-(Acetyloxy)-5-[2-dimethylamino)ethyl]-2,3-dihydro-2-(4methoxy phenyl)- 1,5benzothiazepin-4(5H)-one D 98%View Details
42399-41-7 (2S-Cis)-3-(Acetyloxy)-5-[2-dimethylamino)ethyl]-2,3-dihydro-2-(4methoxy phenyl)- 1,5benzothiazepin-4(5H)-one D 98%View Details
42399-41-7 -
 42399-41-7 98%View Details
42399-41-7 98%View Details
42399-41-7 -
 Diltiazem 98%View Details
Diltiazem 98%View Details -
 Diltiazem 98%View Details
Diltiazem 98%View Details -
 Diltiazem 98%View Details
Diltiazem 98%View Details
42399-41-7 -
 Diltiazem Api powderView Details
Diltiazem Api powderView Details
42399-41-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
