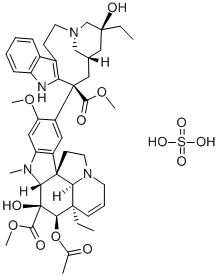
Vinorelbine
- CAS NO.:71486-22-1
- Empirical Formula: C45H54N4O8
- Molecular Weight: 778.93
- MDL number: MFCD00866248
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
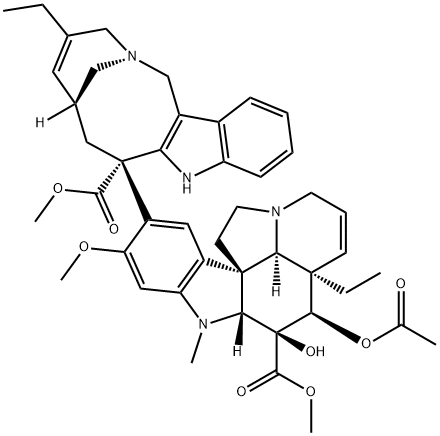
What is Vinorelbine?
Absorption
Vinorelbine is rapidly absorbed with peak serum concentration reached within 2 hours .
Vinorelbine is highly bound to platelets and lymphocytes and is also bound to alpha 1-acid glycoprotein, albumin, and lipoproteins .
Toxicity
Due to the wide array of adverse effects of this drug, the toxicity of is categorized into organ systems .
Hematologic: Granulocytopenia was the primary dose-limiting toxicity with vinorelbine tartrate therapy; it is generally reversible and not cumulative. In one study, granulocytopenia resulted in hospitalizations for fever and/or sepsis in 8% of NSCLC and 9% of breast cancer patients .
Infectious (septic) deaths occurred in about 1% of patients. Grade 3 or 4 anemia occurred in about 1% of lung cancer and approximately 14% of breast cancer patients. Blood transfusions were administered to 18% of patients who received vinorelbine tartrate therapy. The incidence of Grade 3 and 4 thrombocytopenia was found to be less than 1% .
Neurologic: Mild to moderate peripheral neuropathy may occur. Symptoms of paresthesia and hypesthesia are reported as the most commonly reported neurologic toxicities of this drug. The loss of deep tendon reflexes (DTR) occurs in less than 5% of patients, according to one study. The development of severe peripheral neuropathy is rare .
Dermatologic: Alopecia has been reported in only about 12% of patients and is usually reported as mild. Vinorelbine tartrate is a moderate vesicant, leading to injection site reactions. Symptoms include erythema, pain at the injection site and vein discoloration occurred in about 1/3 of all patients. Chemical phlebitis along the vein, near the site of injection, has been reported .
Respiratory: Shortness of breath was reported in 3% of NSCLC and 9% of breast cancer patients, and was severe in 2% of each patient population. Interstitial pulmonary changes have been documented in a few patients .
Gastrointestinal: Mild or moderate nausea symptoms occurred in 32% of NSCLC and 47% of breast cancer patients treated with vinorelbine tartrate. Severe nausea was occurred infrequently (1% and 3% in NSCLC and breast cancer patients, respectively). Prophylactic administration of anti-emetics was not routine in patients treated with single-agent vinorelbine tartrate. Constipation occurred in about 28% of NSCLC and 38% of breast cancer patients. The paralytic ileus incidence of less than 2% of patients. Vomiting, diarrhea, anorexia and stomatitis were found to be mild or moderate and occurred in less than 20% of study patients .
Hepatic: Transient elevations of liver enzymes were reported without clinical symptoms.
Cardiovascular: Chest pain was reported in 5% of NSCLC and 8% of breast cancer patients. Most reports of chest pain were in patients who had either a history of cardiovascular disease or tumor within the chest. There have been rare reports of myocardial infarction; however, these have not been shown definitely attributable to vinorelbine tartrate .
Other: Muscle weakness (asthenia) occurred in about 25% of patients with NSCLC and 41% of patients with breast cancer. It was usually mild or moderate but showed a linear increase with cumulative doses .
Several other toxicities reported in approximately 5% of patients include jaw pain, myalgia, arthralgia, headache, dysphagia, and skin rash. Hemorrhagic cystitis (bladder inflammation with blood in urine) and the syndrome of inappropriate ADH secretion were both reported in less than 1% of patients. The treatment of these entities are mainly symptomatic .
The carcinogenic potential of Vinorelbine has not been adequately studied. Vinorelbine has been demonstrated to affect chromosome number and likely the chromosome structure in vivo (polyploidy in bone marrow cells from Chinese hamsters and a positive micronucleus test in mice were observed) .
Description
Vinorelbine is a semisynthetic vinca alkaloid differing from vinblastine in the catharantine moiety of the molecule. It is claimed to have a broad spectrum of action both in vifro and in vivo; clinically it has been found effective in the treatment of non-small cell lung cancer, advanced breast cancer, ovarian cancer and Hodgkins disease.
Originator
CNRS (France)
The Uses of Vinorelbine
antimigraine, 5HT[1B/1D] agonist
The Uses of Vinorelbine
Vinorelbine base is an antineoplastic agent with anti-mitotic properties.
Indications
Vinorelbine tartrate is indicated for adults in the treatment of advanced non-small cell lung cancer (NSCLC), as a single therapy or in combination with other chemotherapeutic drugs .
Used in relapsed or refractory Hodgkin lymphoma, in combination with other chemotherapy agents .
For the treatment of desmoid tumor or aggressive fibromatosis, in combination with methotrexate .
For the treatment of recurrent or metastatic squamous cell head and neck cancer .
For the treatment of recurrent ovarian cancer .
For the treatment of metastatic breast cancer, in patients previously treated with anthracyline and/or taxane therapy .
For the treatment of HER2-positive, trastuzumab-resistant, advanced breast cancer in patients previously treated with a taxane, in combination with trastuzumab and everolimus .
Background
Vinorelbine is an anti-mitotic chemotherapy drug that is used in the treatment of several types of malignancies, including breast cancer and non-small cell lung cancer (NSCLC) . It was initially approved in the USA in 1990's for the treatment of NSCLC .
It is a third-generation vinca alkaloid. The introduction of third-generation drugs (vinorelbine, gemcitabine, taxanes) in platinum combination improved survival of patients with advanced NSCLC, with very similar results from the various drugs. Treatment toxicities are considerable in the combination treatment setting .
A study was done on the clearance rate of vinorelbine on individuals with various single polymorphonuclear mutations. It was found that there was 4.3-fold variation in vinorelbine clearance across the cohort, suggesting a strong influence of genetics on the clearance of this drug .
What are the applications of Application
Vinorelbine base is an antineoplastic agent with anti-mitotic properties
Definition
ChEBI: A vinca alkaloid with a norvinblastine skeleton.
Manufacturing Process
(+/-)-5-Ethyl-1,2,3,6-tetrahydro-pyridine-3-carboxylic acid ethyl ester and
(+)-tartaric acid were dissolved in hot 95% ethanol. The resulting solution
was allowed to slowly cool to room temperature and refrigerated overnight.
The crystals were filtered, washed with cold ethanol, and recrystallized from
95% ethanol, cooling as before to give the (+)-tartrate salt of (+)-5-ethyl-
1,2,3,6-tetrahydro-pyridine-3-carboxylic acid ethyl ester.
The salt of (+)-5-ethyl-1,2,3,6-tetrahydro-pyridine-3-carboxylic acid ethyl
ester was dissolved in on ice, and 3 N sodium hydroxide was slowly added
until the pH reached 11-12. The solution was extracted with chloroform, dried
(Na 2 SO 4 ) and evaporated in vacuum to give (+)-5-ethyl-1,2,3,6-tetrahydro-
pyridine-3-carboxylic acid ethyl ester as a mobile oil.
Reduction of (+)-5-ethyl-1,2,3,6-tetrahydro-pyridine-3-carboxylic acid ethyl
ester with LiAlH 4 in THF, using the usual protocols associated with this reagent
gave the (+)-5-ethyl-1,2,3,6-tetrahydro-pyridine-3-ol. This material was used
directly in the next step.
To a solution of the (+)-5-ethyl-1,2,3,6-tetrahydro-pyridine-3-ol in ethanol
and triethylamine water, cooled equiv.) was added allyl bromide and the
mixture heated at reflux for 12 h. The mixture was evaporated in vacuo and
the residue dissolved in chloroform and washed with 5% aqueous K 2 CO 3 . The
chloroform layer was dried (MgSO 4 ) and evaporated in vacuo to give the (+)-
1-allyl-5-ethyl-1,2,3,6-tetrahydro-pyridin-3-ol.
The (+)-1-allyl-5-ethyl-1,2,3,6-tetrahydro-pyridin-3-ol was converted into its
methanesulfonate ester in the standard manner by treatment with
methanesulfonic acid.
To a solution of the (+)-1-allyl-5-ethyl-1,2,3,6-tetrahydro-pyridin-3-methanesulfonate in acetone was added lithium bromide (6.9 g, 0.08 mol)
and the suspension heated at reflux. The acetone was evaporated in vacuo,
and the residue partitioned between chloroform and cold aqueous 5% K 2 CO 3
solution. The chloroform layer was dried (MgSO 4 ), filtered, and evaporated in
vacuo to give a brown oil. Fractional distillation gave the (+)-1-allyl-5-ethyl-
1,2,3,6-tetrahydro-pyridin-3-bromide.
To a solution of N-phenylsulfonyl indole (5.14 g, 20 mmol) in dry THF (100
ml) under argon, and cooled to -65°C., was added t-butyl lithium (13 ml, 22
mmol, 1.7 M in pentane). The solution was allowed to warm to 0°C and
stirred for 1 h. The above solution was added via canula to a stirred solution
of dimethyloxalate (9.5 g, 80 mmol) in THF (250 ml) at 0°C. After 4 h at 0°C
the mixture was quenched with saturated aqueous NH 4 Cl and extracted with
ethyl acetate (3 times 100 ml). The dried (MgSO 4 ) extract was evaporated in
vacuum, and the residue purified by chromatography over silica gel eluting
with hexane/ethyl acetate (b 10:1) to give the N-phenylsulfonyl-2-methoxalyl
indole (2.3 g, 34%). Melting point 111°-112°C (from ethyl acetate).
To the (+)-1-allyl-5-ethyl-1,2,3,6-tetrahydro-pyridin-3-bromide in a flame
dried flask under argon was added Mg powder and dry THF. The mixture was
heated at reflux and two drops of 1,2-dibromoethane added to initiate
Grignard reagent formation. After 3 h the turbid suspension was cooled to
room temperature and added to a solution of the N-phenylsulfonyl-2-
methoxalyl indole in THF, at 0°C under argon. After 30 min the solution was
quenched with saturated aqueous NH 4 Cl solution, and diluted with ethyl
acetate. The dried (MgSO 4 ) extract was evaporated in vacuo to give the (+)-
methyl 2-[2-(N-phenylsulfonyl)indolyl]-2-hydroxy-3-[3-(N-allyl 5-ethyl-
1,2,3,6-tetrahydro-peridine)]propionate consisting of a mixture of
diastereomers at C-2 (1:1). For the purpose of characterization, one of the
diastereomers was purified by chromatography over silica gel eluting with
hexane/ethyl acetate/10% aqueous NH 4 OH/MeOH (15:3:1) to give the (+)-
methyl 2-[2-(N-phenylsulfonyl)indolyl]-2-hydroxy-3-[3-(N-allyl-5-ethyl-
1,2,3,6-tetrahydro-pyridine)]propionate.
To a solution of the (+)-methyl 2-[2-(N-phenylsulfonyl)indolyl]-2-hydroxy-3-
[3-(N-allyl-5-ethyl-1,2,3,6-tetrahydropyridine)]propionate (a mixture of
diastereomers at C-2) in dry dimethoxyethane at -50°C under argon was
added sodium naphthalenide (1 M in THF). The mixture was quenched with
trifluoroacetic acid, and extracted with ethyl acetate (3 times 10 ml). The
extract was washed with saturated aqueous NaHCO 3 solution, dried (MgSO 4 )
and evaporated in vacuo to give the (+)-methyl 2-(2-indolyl)-2-hydroxy-3-[3-
(N-allyl-5-ethyl-1,2,3,6-tetrahydro-pyridine )]propionate. The mixture of
diastereomers was not separated but chromotagraphed over silica gel eluting
with hexane/ethyl acetate/10% aqueous NH 4 OH/MeOH (5:1:1) to remove
more polar impurities.
A solution of the (+)-methyl 2-(2-indolyl)-2-hydroxy-3-[3-(N-allyl-5-ethyl-
1,2,3,6-tetrahydro-pyridine)]propionate (mixture of diastereomers), and
vindoline in 1% HCl/MeOH was heated at reflux for 2 h. The solution was
evaporated in vacuo and the residue dissolved in chloroform and washed with
saturated aqueous NaHCO 3 solution. The chloroform layer was dried over
MgSO 4 , filtered, and evaporated to give a foam consisting of a mixture of S-and R-diastereomers. The diastereomeric mixture was separated by
preparative HPLC eluting with hexane/CH 2 Cl 2 /MeOH/10% aqueous NH 4 OH to
give S-(+)-4-acethoxy-9-[2-(1-allyl-5-ethyl-1,2,3,6-tetrahydro-pyridin-3-yl)-1-
(1H-indol-2-yl)-1-methoxycarbonyl-ethyl]-3a-ethyl-5-hydroxy-8-methoxy-6-
methyl-3a,4,5,5a,6,11,12,12b-octahydro-1H-6,12a-diaza-indeno[7,1-
ca]fluorene-5-carboxylic acid methyl ester.
The S-(+)-4-acethoxy-9-[2-(1-allyl-5-ethyl-1,2,3,6-tetrahydro-pyridin-3yl)-1-
(1H-indol-2-yl)-1-methoxycarbonyl-ethyl]-3a-ethyl-5-hydroxy-8-methoxy-6-
methyl-3a,4,5,5a,6,11,12,12b-octahydro-1H-6,12a-diaza-indeno[7,1-
ca]fluorene-5-carboxylic acid methyl ester in 1,2-dichloroethane, containing
proton sponge at 25°C, was treated with 1-chloroethyl chloroformate (0.056
ml, 0.512 mmol, 2.0 equiv.) and the resulting solution stirred for 3 h. The
mixture was evaporated in vacuo, and the residue dissolved in methanol and
heated at reflux for 3 h. The methanol was evaporated and the residue
dissolved in chloroform and purified by chromatography over silica gel eluting
with CHCl 3 , MeOH, 10% aqueous NH 4 OH (20:1) to give S-(+)-4-acethoxy-9-
[2-(5-ethyl-1,2,3,6-tetrahydro-pyridin-3yl)-1-(1H-indol-2-yl)-1-
methoxycarbonyl-ethyl]-3a-ethyl-5-hydroxy-8-methoxy-6-methyl-
3a,4,5,5a,6,11,12,12b-octahydro-1H-6,12a-diaza-indeno[7,1-ca]fluorene-5-
carboxylic acid methyl ester.
To a solution of the S-(+)-4-acethoxy-9-[2-(5-ethyl-1,2,3,6-tetrahydro-
pyridin-3yl)-1-(1H-indol-2-yl)-1-methoxycarbonyl-ethyl]-3a-ethyl-5-hydroxy-
8-methoxy-6-methyl-3a,4,5,5a,6,11,12,12b-octahydro-1H-6,12a-diaza-
indeno[7,1-ca]fluorene-5-carboxylic acid methyl ester in dioxane and glacial
acetic acid was added 37% aqueous formaldehyde and the mixture stirred at
35°C for 24 h. The solution was evaporated in vacuo and the residue
suspended in chloroform and washed with cold aqueous 5% K 2 CO 3 solution.
The chloroform layer was dried (MgSO 4 ), filtered, and evaporated. The residue
was chromatographed eluting with EtOAc/MeOH, 10% NH 4 OH to give the
product navelbine.
brand name
Navelbine (Pierre).
Therapeutic Function
Antineoplastic
Pharmacokinetics
Vinorelbine is a semi-synthetic vinca-alkaloid with a wide spectrum of anti-tumor activity. The vinca-alkaloids are considered spindle poisons. They work by interfering with the polymerization of tubulin, a protein responsible for building the microtubule system which appears during cell division in proliferating cancer cells .
Pharmacokinetics
This semisynthetic alkaloid is unique in having oral bioavailability, but it currently is available only for IV injection. The initial phase elimination half-life is on par with that observed for vincristine and vinblastine, and the terminal phase half-life is between 28 and 44 hours.
Clinical Use
Vinorelbine is particularly useful in the treatment of advanced non–small cell lung cancer and can be administered alone or in combination with cisplatin. It is thought to interfere with mitosis in dividing cells through a relatively specific action on mitotic microtubules.
Side Effects
Although dose-limiting granulocytopenia is the major adverse effect, potentially fatal interstitial pulmonary changes have been noted, and patients with symptoms of respiratory distress should be promptly evaluated. As with all vinca alkaloids, elimination is primarily hepatobiliary, and dosage reduction should be considered in patients with liver dysfunction.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: increased risk of neutropenia with
clarithromycin; possible increased risk of ventricular
arrhythmias with delamanid.
Antifungals: metabolism possibly inhibited by
itraconazole, increased risk of neurotoxicity.
Antimalarials: avoid with piperaquine with
artenimol.
Antipsychotics: avoid concomitant use with
clozapine (increased risk of agranulocytosis).
Metabolism
Metabolism of vinorelbine appears to be hepatic.
All metabolites of vinorelbine are formed by the
CYP3A4 isoform of cytochromes P450, except
4-O-deacetylvinorelbine which is likely to be formed by
carboxylesterases.
4-O-deacetylvinorelbine is the only active metabolite and
the main one observed in blood. Excretion is mainly by
the biliary route (18.5
% appears in the urine).
Metabolism
Vinorelbine undergoes substantial hepatic elimination in humans. Two metabolites of vinorelbine have been identified in human blood, plasma, and urine; vinorelbine N-oxide and deacetylvinorelbine. Deacetylvinorelbine has been demonstrated to be the primary metabolite of vinorelbine in humans, and has been shown to possess antitumor activity similar to vinorelbine , .
Vinorelbine is metabolized into two other minor metabolites, 20'-hydroxyvinorelbine and vinorelbine 6'-oxide .
Therapeutic doses of vinorelbine (30 mg/m2) yield very small, if any, quantifiable levels of either metabolite in blood or urine. The metabolism of vinorelbine is mediated by hepatic cytochrome P450 isoenzymes in the CYP3A subfamily , .
As the liver provides the main route for metabolism of the drug, patients with hepatic impairment may demonstrate increased toxicity with standard dosing, however, there are no available data on this. Likewise, the contribution of cytochrome P450 enzyme action to vinorelbine metabolism has potential implications in patients receiving other drugs metabolized by this route .
Properties of Vinorelbine
| alpha | D20 +52.4° (c = 0.3 in CHCl3) |
| Density | 1.36±0.1 g/cm3(Predicted) |
| storage temp. | Store at 4°C, protect from light |
| solubility | >25.9mg/mL in DMSO |
| form | Powder |
| pka | 11.36±0.60(Predicted) |
Safety information for Vinorelbine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Vinorelbine
Vinorelbine manufacturer
Venkatasai Life Sciences
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 71486-22-1 Vinorelbine 99%View Details
71486-22-1 Vinorelbine 99%View Details
71486-22-1 -
 71486-22-1 98%View Details
71486-22-1 98%View Details
71486-22-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1