Silybin
Synonym(s):(2R,3R)-3,5,7-Trihydroxy-2-[3-(4-hydroxy-3-methoxyphenyl)-2-hydroxymethyl-2,3-dihydrobenzo[1,4]dioxin-6-yl]chroman-4-one;2,3-Dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-6-(3,5,7-trihydroxy-4-oxobenzopyran-2-yl)benzodioxin;flavolignans;Silibinin;Silybin
- CAS NO.:22888-70-6
- Empirical Formula: C25H22O10
- Molecular Weight: 482.44
- MDL number: MFCD00872186
- EINECS: 245-302-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:55
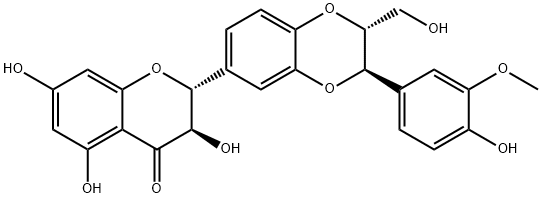
What is Silybin?
Description
Silybin, also known as silibinin, is the principal biologically active constituent of the silymarin complex (about 70–80%) and is a mixture of silybin A and silybin B. Pharmacological research demonstrated that silybin has shown pleiotropic biological properties, including potent antioxidant, anti-inflammatory, anticancer, antidiabetic, hepatoprotective, neuroprotective, antiviral, antimicrobial and immunosuppressive activities. Silibinin demonstrates promising anticancer effects in vitro and in vivo[1].
Chemical properties
solid
Originator
Legalon,Madaus,W. Germany,1969
The Uses of Silybin
Silibinin has been used:
- to study its effect on gene expression levels of various proteins involved in chromatin regulations of prostate cancer, by real time polymerase chain reaction (RT-PCR)
- to study its effect on cell proliferation in platelet-derived growth factor (PDGF)-treated human tenon′s fibroblasts (HTFs) by investigating the expression of proliferating cell nuclear antigen (PCNA) and by water-soluble tetrazolium salt (WST-1) assay
- to examine its effect on gene expression levels of stromelysine 1 (STM1), acetyl hexoseamines and collagen production during skin wound healing
- to study its inhibitory effect on Escherichia coli ATP synthase
The Uses of Silybin
Labelled Silybin (S465850). Hepatoprotectant.
What are the applications of Application
Silybin is a major active constituent of silymarin which inhibits mitogen-activated protein kinase (MAPK)
Definition
ChEBI: A flavonolignan isolated from milk thistle, Silybum marianum, that has been shown to exhibit antioxidant and antineoplastic activities.
Manufacturing Process
Silymarin comprising polyhydroxyphenyl chromanones is recovered from the dried fruit of Silybum marianum Gaertn. by separating the fatty oils therefrom, extracting the remaining solid residue with ethyl acetate, evaporating the ethyl acetate and dissolving the dry residue in a solvent mixture comprising methanol, water and petroleum ether to form a two-phase system wherein the chromanones are contained in the lower phase, recovering the polyhydroxyphenyl chromanones from the lower phase after subjecting same to multiple counter-current contact with petroleum ether.
Therapeutic Function
Hepatoprotectant
Biological Functions
Silybin has excellent therapeutic effects on EAE by inhibiting the polarization of Th1. Moreover, it has been proven to protect pancreatic β-cells, attenuate hepatic glucose production, and increase glucose uptake in muscle in vitro and in vivo. Glucokinase (GK) and PPARγ are essential targets for antidiabetic use. Silybin was an agonist of GK and PPARγ. It could improve glycemic homeostasis by improving the activity of pancreatic β,-cells, increasing insulin sensitivity of liver and muscle cells, and decreasing lipid deposition in adipocytes. The molecular mechanisms of silibinin-mediated antiproliferative effects are mainly via receptor tyrosine kinases, androgen receptors, STATs, NF-&kappa, B, cell cycle arrest, and apoptosis/autophagy-inducing signaling pathways in various cancer cells. Next to modulating the NF&kappa signaling pathway, Sibilin inhibits NLRP1/NLRP3 inflammasome signaling pathways. It modulates many molecular changes caused by xenobiotics and ultraviolet radiation to protect the skin and is used in cosmeceutical preparations[2].
General Description
Silibinin, a principal component of silymarin, is a flavonolignan and an orally active flavonoid. It is isolated from the seeds of milk thistle (Sylibum marianum L).
Biochem/physiol Actions
Silibinin (Silybin) functions as a hepatoprotectant in patients with acute and chronic liver injury. This flavonoid fights against various cancer cells including, prostate, skin, breast, colon, lung, bladder and hepatocellular carcinoma (HCC). Silibinin exhibits its efficacy as an anti-cancer agent by blocking the secretion of proangiogenic factors from tumor cells, and by hindering growth and inducing apoptotic death of endothelial cells. In addition, it also interrupts capillary tube formation on Matrigel. Silibinin might be a good candidate for chemoprevention of prostate cancer (PC). It might be used as a potential therapeutic regimen for the treatment of endometriosis in vitro and in vivo.
References
[1] Huan-Li Yang, Xiao-Wu Shi. “Silybin Alleviates Experimental Autoimmune Encephalomyelitis by Suppressing Dendritic Cell Activation and Th17 Cell Differentiation.” Frontiers in Neurology (2021): 659678.
[2] Zhipeng Zhang. “Discovery of Potent Glucokinase and PPARγ Dual-Target Agonists through an Innovative Scheme for Regioselective Modification of Silybin.” ACS Omega 7 4 (2022): 3812–3822.
[3] Romanucci, Valeria et al. “7-O-tyrosyl Silybin Derivatives as a Novel Set of Anti-Prostate Cancer Compounds.” Antioxidants 38 1 (2024).
Properties of Silybin
| Melting point: | 164-174°C |
| Boiling point: | 793.0±60.0 °C(Predicted) |
| alpha | D20 +11° (c = 0.25 in acetone + alcohol) |
| Density | 1.527±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Acetone (Sparingly), DMSO, Methano (Sparingly) |
| form | Solid |
| pka | pKa 6.42±0.04 (Uncertain) |
| color | Pale Yellow |
| Water Solubility | 54mg/L(24.99 ºC) |
| Merck | 13,8607 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 22888-70-6(CAS DataBase Reference) |
Safety information for Silybin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Silybin
Silybin manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran






You may like
-
 Silymarine 80% 99%View Details
Silymarine 80% 99%View Details -
 U0126-Etoh 95% CAS 22888-70-6View Details
U0126-Etoh 95% CAS 22888-70-6View Details
22888-70-6 -
 Silibinin, ≥98% (HPLC) CAS 22888-70-6View Details
Silibinin, ≥98% (HPLC) CAS 22888-70-6View Details
22888-70-6 -
 Silybin 98% CAS 22888-70-6View Details
Silybin 98% CAS 22888-70-6View Details
22888-70-6 -
 Silybin CAS 22888-70-6View Details
Silybin CAS 22888-70-6View Details
22888-70-6 -
 Silybin CAS 22888-70-6View Details
Silybin CAS 22888-70-6View Details
22888-70-6 -
 Silibinin CAS 22888-70-6View Details
Silibinin CAS 22888-70-6View Details
22888-70-6 -
 Silibinin Cas 22888 70 6View Details
Silibinin Cas 22888 70 6View Details
22888-70-6
