Vanillylacetone
Synonym(s):4-(4-Hydroxy-3-methoxyphenyl)-2-butanone;Vanillyl acetone;Vanillylacetone;Zingerone
- CAS NO.:122-48-5
- Empirical Formula: C11H14O3
- Molecular Weight: 194.23
- MDL number: MFCD00048232
- EINECS: 204-548-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
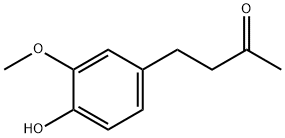
What is Vanillylacetone?
Description
Gingerol (correctly, [6]-gingerol) is the predominant phenol and most important of the pungent constituents in ginger oil. It was isolated by J. C. Thresh in 1879 from the rhizome of the ginger plant (Zingiber officinale). It and its dehydrated analogue [6]-shogaol are the primary ginger-derived bioactive compounds. Shogaol and the fragmented molecule zingerone are produced when fresh ginger is heated or cooked.?A recently synthesized azagingerol analogue?increases metabolism in mice and may reduce the risk of obesity-associated diseases.
Zingerone gives ginger a "hot" taste. It''s also an antioxidant, although it only weakly inhibits peroxidation of phospholipid liposomes in the presence of Fe(III) and ascorbate. Zingerone''s vanillin foundation and hydrocarbon tail make it a chemical relative of?eugenol?and capsaicin.
Chemical properties
White solid. Soluble in ether; sparingly soluble in water and petroleum ether.
Chemical properties
Zingerone has a strong, pungent odor reminiscent of ginger. It has a sharp taste, similar to ginger.
Occurrence
Reported found in the essential oil of Zingiber officinale. Also reported found in cranberry, raspberry, ginger and mango.
The Uses of Vanillylacetone
Vanillylacetone is a phenolic compound that naturally occurs in cranberry and ginger. Studies shows that Vanillylacetone exhibits variable cytotoxic, cytoprotective and antioxidant activity against li ver and human tumor cells. Vanillylacetone is also used in herbal medicine for various purposes.
The Uses of Vanillylacetone
In fragrances, flavors and cosmetics; in artificial spice oils.
The Uses of Vanillylacetone
Vanillylacetone is a phenolic compound that naturally occurs in cranberry and ginger. Studies shows that Vanillylacetone exhibits variable cytotoxic, cytoprotective and antioxidant activity against liver and human tumor cells. Vanillylacetone is also used in herbal medicine for various purposes.
Definition
ChEBI: A ketone that is 4-phenylbutan-2-one in which the phenyl ring is substituted at positions 3 and 4 by methoxy and hydroxy groups respectively. The major pungent component in ginger.
Preparation
By condensation of vanillin with acetone followed by hydrogenation.
Aroma threshold values
Aroma characteristics at 10.0%: low impacting, creamy, spicy eugenol clove-like with a slight balsamic vanilla-like note.
Taste threshold values
Taste characteristics at 80 ppm: spicy with a biting, lingering heat. Taste characteristics at 20 ppm in 5% sugar solution: smooth, sweet, creamy and warm, spicy clove with a slight lingering burning bite.
Safety Profile
Moderately toxic by ingestion. A skin irritant. A flammable liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
Properties of Vanillylacetone
| Melting point: | 40-41 °C(lit.) |
| Boiling point: | 141 °C0.5 mm Hg(lit.) |
| Density | 1.14 g/mL at 25 °C(lit.) |
| refractive index | n |
| FEMA | 3124 | ZINGERONE |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Acetonitrile (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 10.03±0.20(Predicted) |
| form | Solid |
| color | White to Pale Yellow Low-Melting |
| Odor | at 100.00 %. sweet spicy phenolic ginger vanilla woody |
| Merck | 14,10166 |
| JECFA Number | 730 |
| CAS DataBase Reference | 122-48-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Butanone, 4-(4-hydroxy-3-methoxyphenyl)-(122-48-5) |
| EPA Substance Registry System | 2-Butanone, 4-(4-hydroxy-3-methoxyphenyl)- (122-48-5) |
Safety information for Vanillylacetone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Vanillylacetone
| InChIKey | OJYLAHXKWMRDGS-UHFFFAOYSA-N |
Vanillylacetone manufacturer
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


![2,10-DIAZA-9-(4-HYDROXY-3,5-DIMETHOXYPHENYL)-5,5-DIMETHYLTRICYCLO[9.4.0.0(3,8)]PENTADECA-1(11),3(8),12,14-TETRAEN-7-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB3776300.gif)
![[2-(BENZYLOXY)PHENYL][3-(3,4,5-TRIMETHOXYPHENYL)OXIRAN-2-YL]METHANONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB8321738.gif)
![2,9-DIAZA-10-BENZO[D]1,3-DIOXOLEN-5-YL-14,14-DIMETHYL-12-OXOTRICYCLO[9.4.0.0(3,8)]PENTADECA-1(11),3(8),4,6-TETRAENE-5-CARBOXYLIC ACID](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB1757761.gif)
You may like
-
 122-48-5 Zingiberone 98%View Details
122-48-5 Zingiberone 98%View Details
122-48-5 -
 4-(4-Hydroxy-3-methoxyphenyl)-2-butanone CAS 122-48-5View Details
4-(4-Hydroxy-3-methoxyphenyl)-2-butanone CAS 122-48-5View Details
122-48-5 -
 Vanillylacetone 95% CAS 122-48-5View Details
Vanillylacetone 95% CAS 122-48-5View Details
122-48-5 -
 Zingerone 98.00% CAS 122-48-5View Details
Zingerone 98.00% CAS 122-48-5View Details
122-48-5 -
 Zingerone CAS 122-48-5View Details
Zingerone CAS 122-48-5View Details
122-48-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1