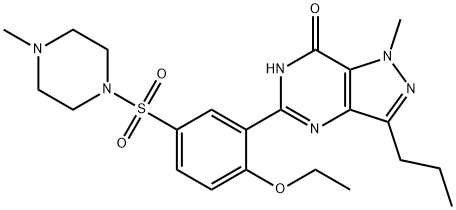
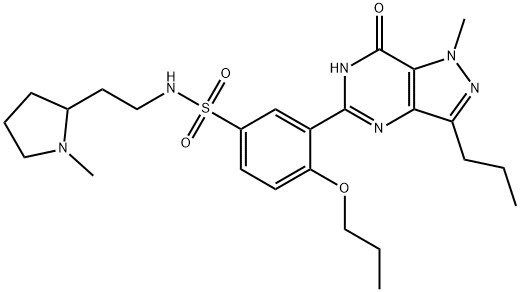
Udenafil
Synonym(s):3-(1-Methyl-7-oxo-3-propyl-4,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-N-[2-(1-methylpyrrolidin-2-yl)ethyl]-4-propoxybenzenesulfonamide;3-(4,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-N-[2-(1-methyl-2-pyrrolidinyl)ethyl]-4-propoxybenzenesulfonamide;3-(6,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-N-[2-(1-methyl-2-pyrrolidinyl)ethyl]-4-propoxybenzenesulfonamide;5-[2-Propoxy-5-(1-methyl-2-pyrrolidinylethyl amidosulfonyl)phenyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo(4,3-d)pyrimidin-7-one;5-[2-Propyloxy-5-(1-methyl-2-pyrrolidinylethylamidosulfonyl)phenyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo(4,3-d)pyrimidin-7-one
- CAS NO.:268203-93-6
- Empirical Formula: C25H36N6O4S
- Molecular Weight: 516.66
- MDL number: MFCD20275557
- EINECS: 251-228-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Udenafil?
Description
Udenafil is the fourth in a class of drugs targeting the inhibition of the enzyme phosphodiesterase 5 (PDE5) for the treatment of erectile dysfunction. Inhibition of PDE5 results in the increase in endogenous cyclic guanosine monophosphate (cGMP) concentrations in the penile corpus cavernosum. cGMP induces smooth muscle cell relaxation and subsequent increased blood flow leading to a sustainable erection. Udenafil is a potent antagonist of human PDE5 with an IC50 of 8.25nM and a comparable selectivity profile as sildenafil for the other PDEs. Unlike tadalafil, it does not inhibit PDE11, which has been implicated in myalgia and testicular toxicity. Furthermore, udenafil produced up to a 91% vaginal penetration success rate and up to a 67% intercourse completion rate compared to a 29% completion rate by placebo. Overall patient satisfaction, measured by a standard global assessment question, was 86% compared to only 26% in the placebo group. The most frequently recorded adverse events were mild-to-moderate facial flushing and headache.
Description
Udenafil is an inhibitor of phosphodiesterase 5 (PDE5). In vivo, udenafil (1 and 5 mg/kg) increases lung cGMP levels, attenuates the development of compensatory right ventricular hypertrophy, and reduces pulmonary arterial wall thickening in a rat model of monocrotaline-induced pulmonary hypertension. It increases creatine clearance and decreases blood urea nitrogen (BUN) and serum malondialdehyde (MDA) levels in a rat model of renal ischemia-reperfusion injury. Udenafil (0.3 and 10 mg/kg) induces penile erections in conscious rabbits and in rabbits with acute spinal cord injury.
Chemical properties
Off-White Solid
Originator
Dong-A (South Korea)
The Uses of Udenafil
Udenafil is an oral phosphodiesterase 5 inhibitor used for the treatment of erectile dysfunction.
What are the applications of Application
Udenafil is an oral PDE5A (phosphodiesterase 5) inhibitor.
Background
Udenafil is a new phosphodiesterase type 5 (PDE5) inhibitor used to treat erectile dysfunction (ED). It has been approved in South Korea and will be marketed under the brand name Zydena. It is not yet approved for use in the U.S., E.U., or Canada.
Indications
Investigated for use/treatment in erectile dysfunction and hypertension.
Definition
ChEBI: Udenafil is a sulfonamide.
brand name
Zydena
Pharmacokinetics
Udenafil is a potent selective phosphodiesterase type 5 (PDE5) inhibitor.
Synthesis
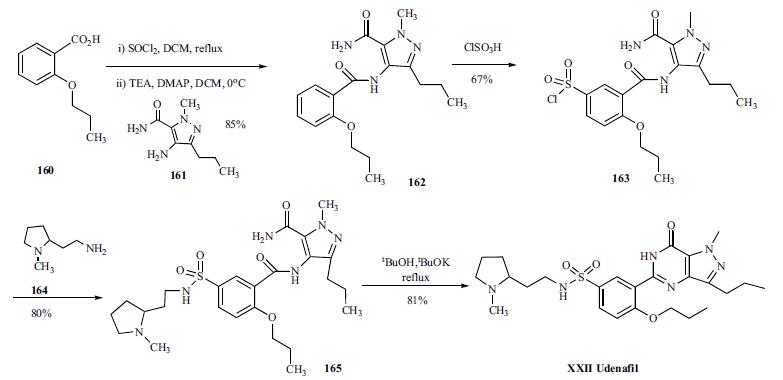
Udenafil has a unique pharmacokinetic profile with a relatively rapid onset and sufficiently long duration (Tmax 1-1.5 hr, t1/2 11-13 hr) to make it effective for up to 24 hours. Synthesis of this racemic compound started with commercially available 2-propoxybenzoic acid (160). The free acid 160 was converted to it acyl chloride with thinoy chloride in refluxing dichloromethane, which was condensed with 4-amino-1-methyl-3- propyl-1H-pyrazole-5-carboxamide (161) with TEA and DMAP in dichloromethane to yield carboxamide 162 in 85% yield from 160. Compound 162 was sulfonated with chlorosulfonic acid to yield benzenesulfonyl chloride 163 in 67% yield, which was treated with racemic 2-(1-methylpyrrolidin- 2-yl)ethylamine (164) in dichloromethane to afford sulfonamide 165 in 80% yield. Finally, compound 165 was cyclized with tBuOK in refluxing tBuOH to give udenafil (XXII) in 81% yield.
Metabolism
Hepatic. Metabolized by CYP3A4 and CYP3A5.
Properties of Udenafil
| Melting point: | 152-159°C |
| Boiling point: | 697.0±65.0 °C(Predicted) |
| Density | 1.35 |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Sparingly) |
| form | Solid |
| pka | 11.07±0.50(Predicted) |
| color | White to Pale Beige |
Safety information for Udenafil
Computed Descriptors for Udenafil
Udenafil manufacturer
Rakshit Group of Companies (Rakshit Drugs Pvt Ltd)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 268203-93-6 Udenafil 98%View Details
268203-93-6 Udenafil 98%View Details
268203-93-6 -
 Udenafil CAS 268203-93-6View Details
Udenafil CAS 268203-93-6View Details
268203-93-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1