Sildenafil
- CAS NO.:139755-83-2
- Empirical Formula: C22H30N6O4S
- Molecular Weight: 474.58
- MDL number: MFCD00867528
- EINECS: 604-158-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is Sildenafil?
Absorption
Sildenafil is known to be quickly absorbed, with maximum plasma concentrations being observed within 30-120 minutes (with a median of 60 minutes) of oral administration in a fasting patient . Moreover, the mean absolute bioavailability observed for sildenafil is about 41% (from a range of 25-63%) . In particular, after oral three times a day dosing of sildenafil, the AUC and Cmax increase in proportion with dose over the recommended dosage range of 25-100 mg .
When used in pulmonary arterial hypertension patients, however, the oral bioavailability of sildenafil after a dosing regimen of 80 mg three times a day, was on average 43% greater than compared to the lower doses .
Finally, if sildenafil is administered orally with food, the rate of absorption is observed to be decreased with a mean delay in Tmax of about 60 minutes and a mean decrease in Cmax of approximately 29% . Regardless, the extent of absorption is not observed to be significantly affected as the recorded AUC decreased by only about 11 % .
Toxicity
In single-dose volunteer studies of doses up to 800 mg, adverse reactions were similar to those seen at lower doses, but the incidence rates and severities were increased . Doses of 200 mg did not result in increased efficacy but the incidence of adverse reaction (headache, flushing, dizziness, dyspepsia, nasal congestion, altered vision) was increased .
Due to the lack of data on the effect of sildenafil indicated for the treatment of pulmonary arterial hypertension (PAH) in pregnant women, sildenafil is not recommended for women of childbearing potential unless also using appropriate contraceptive measures .
The safety and efficacy of sildenafil indicated for treating PAH in a woman during labor and delivery have not been studied . Caution should ultimately be exercised when sildenafil is administered to nursing women as it is not known if sildenafil or its metabolites are excreted in human breast milk .
The safety and efficacy of sildenafil for the treatment of PAH in children below 1 year of age has not been established as no data is available .
Clinical experience with the elderly population in the use of sildenafil for the treatment of PAH has been varied. Some reports suggest that there are no identified differences in responses between elderly and younger patients while others have documented that clinical efficacy as measured by 6-minute walk distance could be less in elderly patients . In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy .
Conversely, when sildenafil was used to treat erectile dysfunction in healthy elderly volunteers (65 years or over), a reduced clearance of sildenafil was observed . This reduction resulted in about 90% higher plasma concentrations of sildenafil and the active N-desmethyl metabolite compared to those seen in healthy younger volunteers (18-45 years) . Due to age-differences in plasma protein binding, the corresponding increase in free sildenafil plasma concentration was approximately 40% .
Sildenafil was not carcinogenic when administered to rats for 24 months at a dose resulting in total systemic drug exposure (AUCs) for unbound sildenafil and its major metabolite of 29- and 42- times, for male and female rats, respectively, the exposures observed in human males given the Maximum Recommended Human Dose (MRHD) of 100 mg . Sildenafil was not carcinogenic when administered to mice for 18-21 months at dosages up to the Maximum Tolerated Dose (MTD) of 10 mg/kg/day, approximately 0.6 times the MRHD on a mg/m2 basis .
Sildenafil was negative in in vitro bacterial and Chinese hamster ovary cell assays to detect mutagenicity, and in vitro human lymphocytes and in vivo mouse micronucleus assays to detect clastogenicity .
There was no impairment of fertility in rats given sildenafil up to 60 mg/kg/day for 36 days to females and 102 days to males, a dose producing an AUC value of more than 25 times the human male AUC .
Description
Sildenafil was launched as Viagra in the US for the treatment of organic orland psychological male erectile dysfunction (ED). It is an orally bioavailable pyrazolopyrimidinone derivative structurally related to zaprinast, with vasodilating and potential anti-inflammatory activities. Upon oral administration, sildenafil selectively targets and inhibits cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), thereby inhibiting the PDE5-mediated degradation of cGMP found in smooth muscle and increasing cGMP availability. This results in prolonged smooth muscle relaxation in the corpus cavernosum of the penis, thereby causing vasodilation, blood engorgement and a prolonged penile erection.
Description
Sildenafil, marketed as its citrate salt under the trade name Viagra, is the leading drug for treating erectile dysfunction. It was originally developed by Pfizer for treating pulmonary hypertension and angina pectoris. After scientists noticed that it produced penile erections, Pfizer patented it for this use in 1996 and received U.S. Food and Drug Administration approval for sale in 1998.
Chemical properties
Sildenafil citrate is a white to off-white crystalline powder soluble in DMF, acetic acid and slightly soluble in methanol. Solubility of sildenafil citrate is pH dependent and it decreases with increase of pH. pH ranges between 3.7 and 3.8 and the pKa from 8.2 to 9.6.
Originator
Pfizer (UK)
The Uses of Sildenafil
Sildenafil is a phosphodiesterase-5 (PDE5) inhibitor. It is indicated for the treatment of erectile dysfunction (ED). Sildenafil is an orally active selective type 5 cGMP phosphodiesterase inhibitor.
Background
In eliciting its mechanism of action, sildenafil ultimately prevents or minimizes the breakdown of cyclic guanosine monophosphate (cGMP) by inhibiting cGMP specific phosphodiesterase type 5 (PDE5) . The result of doing so allows cGMP present in both the penis and pulmonary vasculature to elicit smooth muscle relaxation and vasodilation that subsequently facilitates relief in pulmonary arterial hypertension and the increased flow of blood into the spongy erectile tissue of the penis that consequently allows it to grow in size and become erect and rigid .
Interestingly enough, it is precisely via this mechanism why sildenafil was at first researched as a potential treatment for angina - or chest pain associated with inadequate blood flow to the heart - before being serendipitously indicated for treating erectile dysfunction in the late 1980s . Nevertheless, it is because of this mechanism that sildenafil is also indicated for treating pulmonary arterial hypertension but is also additionally notorious for interacting with various anti-anginal or anti-hypertensive agents to develop potentially rapid, excessive, and/or fatal hypotensive crises .
Regardless, sildenafil, among a variety of other similar or related PDE5 inhibitors, has become a common and effective treatment for erectile dysfunction and since its formal approval for medical use in the public in 1998 , continues to see millions of prescriptions written for it internationally. Although the medication has historically been most popularly recognized as Pfizer's brand name Viagra, sildenafil is currently available generically and even as a non-prescription over the counter medication in some countries .
Indications
Sildenafil is a phosphodiesterase-5 (PDE5) inhibitor that is predominantly employed for two primary indications:
(1) the treatment of erectile dysfunction ; and
(2) treatment of pulmonary hypertension, where:
a) the US FDA specifically indicates sildenafil for the treatment of pulmonary arterial hypertension (PAH) (WHO Group I) in adults to improve exercise ability and delay clinical worsening . The delay in clinical worsening was demonstrated when sildenafil was added to background epoprostenol therapy . Studies establishing effectiveness were short-term (12 to 16 weeks), and included predominately patients with New York Heart Association (NYHA) Functional Class II-III symptoms and idiopathic etiology (71%) or associated with connective tissue disease (CTD) (25%) ;
b) the Canadian product monograph specifically indicates sildenafil for the treatment of primary pulmonary arterial hypertension (PPH) or pulmonary hypertension secondary to connective tissue disease (CTD) in adult patients with WHO functional class II or III who have not responded to conventional therapy . In addition, improvement in exercise ability and delay in clinical worsening was demonstrated in adult patients who were already stabilized on background epoprostenol therapy ; and
c) the EMA product information specifically indicates sildenafil for the treatment of adult patients with pulmonary arterial hypertension classified as WHO functional class II and III, to improve exercise capacity . Efficacy has been shown in primary pulmonary hypertension and pulmonary hypertension associated with connective tissue disease . The EMA label also indicates sildenafil for the treatment of pediatric patients aged 1 year to 17 years old with pulmonary arterial hypertension . Efficacy in terms of improvement of exercise capacity or pulmonary hemodynamics has been shown in primary pulmonary hypertension and pulmonary hypertension associated with congenital heart disease .
Definition
ChEBI: Sildenafil is a pyrazolo[4,3-d]pyrimidin-7-one having a methyl substituent at the 1-position, a propyl substituent at the 3-position and a 2-ethoxy-5-[(4-methylpiperazin-1-yl)sulfonyl]phenyl group at the 5-position. It has a role as a vasodilator agent and an EC 3.1.4.35 (3',5'-cyclic-GMP phosphodiesterase) inhibitor. It is a pyrazolopyrimidine, a member of piperazines and a sulfonamide.
Indications
Sildenafil (Viagra) is a selective inhibitor of PD-5, an enzyme that inactivates cGMP. Vardenifil (Levitra) is a particularly effective inhibitor of PD-5. It has a shorter onset of action and can be used in smaller doses than sildenafil. Other drugs used in the treatment of ED exert their effects through other biochemical pathways, both central and peripheral.
Preparation
The first synthetic route of sildenafil accomplished the preparation of its pyrazole derivative from ethyl 3-butyrylpyruvate and hydrazine hydrate in acetic acid, followed by the selective N-methylation of the pyrazole ring with dimethyl sulfate. The carboxylic acid was obtained after alkaline hydrolysis was subjected to nitration, followed by treatment with concentrated ammonium hydroxide solution to sequentially deliver the corresponding carboxamide derivative. The nitro group of the mentioned carboxamide derivative was then reduced to an amino group by stannous chloride/hydrochloric acid in ethanol, leading to the formation of the main 4-aminopyrazole structure. Mild amidation of the aminopyrazole derivative by the appropriate benzoyl chloride was performed, followed by cyclization mediated by hydrogen peroxide under basic environment which led to the formation of pyrimidinone heterocycle ring. Chloro-suIphonylation of pyrimidinone derivative imposed selectively on the 50 position of the phenyl ring, led to the aroyl sulfonyl chloride derivative which was then coupled with N-methylpiperazine to afford sildenafil.
synthesis of sildenafil
brand name
Viagra (Pfizer);Segurex.
Therapeutic Function
Vasodilator
Mechanism of action
Sildenafil is readily absorbed after oral administration and reaches peak plasma levels after about an hour. It undergoes hepatic metabolism and has a terminal half-life of about 4 hours.An initial dose of 50 mg is taken about an hour prior to sexual activity to induce penile erection.
Pharmacokinetics
In vitro studies have shown that sildenafil is selective for phosphodiesterase-5 (PDE5) . Its effect is more potent on PDE5 than on other known phosphodiesterases . In particular, there is a 10-times selectivity over PDE6 which is involved in the phototransduction pathway in the retina . There is an 80-times selectivity over PDE1, and over 700-times over PDE 2, 3, 4, 7, 8, 9, 10 and 11 . And finally, sildenafil has greater than 4,000-times selectivity for PDE5 over PDE3, the cAMP-specific phosphodiesterase isoform involved in the control of cardiac contractility .
In eight double-blind, placebo-controlled crossover studies of patients with either organic or psychogenic erectile dysfunction, sexual stimulation resulted in improved erections, as assessed by an objective measurement of hardness and duration of erections (via the use of RigiScan?), after sildenafil administration compared with placebo . Most studies assessed the efficacy of sildenafil approximately 60 minutes post-dose . The erectile response, as assessed by RigiScan?, generally increased with increasing sildenafil dose and plasma concentration . The time course of effect was examined in one study, showing an effect for up to 4 hours but the response was diminished compared to 2 hours .
Sildenafil causes mild and transient decreases in systemic blood pressure which, in the majority of cases, do not translate into clinical effects . After chronic dosing of 80 mg, three times a day to patients with systemic hypertension the mean change from baseline in systolic and diastolic blood pressure was a decrease of 9.4 mmHg and 9.1 mmHg respectively . After chronic dosing of 80 mg, three times a day to patients with pulmonary arterial hypertension lesser effects in blood pressure reduction were observed (a reduction in both systolic and diastolic pressure of 2 mmHg) . At the recommended dose of 20 mg three times a day no reductions in systolic or diastolic pressure were seen .
Single oral doses of sildenafil up to 100 mg in healthy volunteers produced no clinically relevant effects on ECG . After chronic dosing of 80 mg three times a day to patients with pulmonary arterial hypertension no clinically relevant effects on the ECG were reported either .
In a study of the hemodynamic effects of a single oral 100 mg dose of sildenafil in 14 patients with severe coronary artery disease (CAD) (> 70 % stenosis of at least one coronary artery), the mean resting systolic and diastolic blood pressures decreased by 7 % and 6 % respectively compared to baseline . Mean pulmonary systolic blood pressure decreased by 9% . Sildenafil showed no effect on cardiac output and did not impair blood flow through the stenosed coronary arteries .
Mild and transient differences in color discrimination (blue/green) were detected in some subjects using the Farnsworth-Munsell 100 hue test at 1 hour following a 100 mg dose, with no effects evident after 2 hours post-dose . The postulated mechanism for this change in color discrimination is related to inhibition of PDE6, which is involved in the phototransduction cascade of the retina . Sildenafil has no effect on visual acuity or contrast sensitivity. In a small size placebo-controlled study of patients with documented early age-related macular degeneration (n = 9), sildenafil (single dose, 100 mg) demonstrated no significant changes in visual tests conducted (which included visual acuity, Amsler grid, color discrimination simulated traffic light, and the Humphrey perimeter and photostress test) .
Clinical Use
Sildenafil is a selective inhibitor of cGMP-specific PD-5 and therefore inhibits the degradation of cGMP. PD-5, the predominant type in the corpus cavernosum, also is present in other tissues (e.g., lungs, platelets, and eye). The selective inhibition of this enzyme facilitates the release of nitric oxide and smooth muscle relaxation of the corpus cavernosa. Sildenafil enhances erection by augmenting nitric oxide–mediated relaxation pathways. It has been suggested that sildenafil’s mechanism of action is due to cross-talk between cGMP- and cAMPdependent transduction pathways within the cavernous muscles.
Side Effects
Orally administered sildenafil is an effective and well-tolerated treatment for men with ED, including those with diabetes mellitus. It has also been used for so-called salvage therapy in men who do not respond to intracorporeal injections of other agents. Headache is a common side effect, as are flushing and rhinitis.More serious side effects include definite or suspected myocardial infarctions and cardiac arrest.
Enzyme inhibitor
Sildenafil is rapidly absorbed and peaks in concentration (127–560 ng/mL) after 0.5 to 2.0 hours, displaying a half-life of 3 to 4 hours for the full therapeutic dose (25–100 mg). It is 96% bound to plasma proteins and is metabolized by the liver CYP3A4. The metabolite N-desmethylsildenafil possesses approximately 50% of the activity of the parent molecule.
Drug interactions
Potentially hazardous interactions with other drugs
Alpha-blockers: enhanced hypotensive effect - avoid
for 4 hours after sildenafil.
Antibacterials: concentration increased by
clarithromycin and erythromycin - consider
reducing sildenafil dose or frequency.
Antifungals: concentration increased by ketoconazole
- reduce initial dose for ED and avoid for PAH;
concentration increased by itraconazole - reduce
initial dose of sildenafil.
Antivirals: ritonavir significantly increases sildenafil
concentration - avoid; concentration possibly
increased by saquinavir, fosamprenavir and indinavir
- reduce dose of sildenafil; concentration reduced
by etravirine; side effects possibly increased by
atazanavir; increased risk of ventricular arrhythmias
with saquinavir - avoid; avoid with telaprevir; avoid
with tipranavir for PAH.
Cobicistat: concentration of sildenafil possibly
increased - reduce initial dose for ED and avoid for
PAH.
Nicorandil: enhanced hypotensive effect - avoid.
Nitrates: enhanced hypotensive effect - absolutely
contraindicated.
Riociguat: enhanced hypotensive effect - avoid.
Metabolism
The metabolism of sildenafil is facilitated primarily by the CYP3A4 hepatic microsomal isoenzymes and to a minor extent, via the CYP2C9 hepatic isoenzymes . The predominant circulating metabolite results from the N-demethylation of sildenafil . This particular resultant metabolite possesses a phosphodiesterase selectivity that is similar to the parent sildenafil molecule and a corresponding in vitro potency for PDE5 that is approximately 50% that of the parent drug . Moreover, plasma concentrations of the metabolite are about 40% of those recorded for sildenafil, a percentage that accounts for about 20% of sildenafil’s pharmacologic effects . This primary N-desmethyl metabolite of sildenafil also undergoes further metabolism, with a terminal half-life of about 4 hours .
In patients with pulmonary arterial hypertension, plasma concentrations of the primary N-desmethyl metabolite are about 72% those of the original parent sildenafil molecule after a regimen of 20 mg three times a day - which is consequently responsible for about a 36% contribution to sildenafil’s overall pharmacological effects .
Metabolism
In vitro metabolism studies for sildenafil have shown that the primary metabolite, N-desmethylsildenafil, and the minor metabolite, oxidative opening of the piperazine ring, are mediated by CYP3A4, CYP2C9, CYP2C19, and CYP2D6. The estimated relative contributions to clearance were 79% for CYP3A4, 20% for CYP2C9, and less than 2% for CYP2C19 and CYP2D6. These results demonstrate that CYP3A4 is the primary cytochrome mediating N-demethylation and that drugs inhibiting CYP3A4 likely impair sildenafil biotransformation and clearance. The pharmacokinetics of radiolabeled sildenafil were consistent with rapid absorption, first-pass metabolism, and primarily fecal elimination of N-demethylated metabolites. The absorption of sildenafil following oral administration was rapid (~92%), whereas the oral bioavailability was approximately 38% as a result of first-pass metabolism.
Properties of Sildenafil
| Melting point: | 187-189°C |
| Density | 1.39±0.1 g/cm3(Predicted) |
| Flash point: | 9℃ |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 8.7 (Uncertain) |
| color | White to Off-White |
| CAS DataBase Reference | 139755-83-2(CAS DataBase Reference) |
| EPA Substance Registry System | 7H-Pyrazolo[4,3-d]pyrimidin-7-one, 5-[2-ethoxy-5-[(4-methyl-1-piperazinyl)sulfonyl]phenyl]-1,6-dihydro-1-methyl-3-propyl- (139755-83-2) |
Safety information for Sildenafil
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Sildenafil
| InChIKey | BNRNXUUZRGQAQC-UHFFFAOYSA-N |
Sildenafil manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
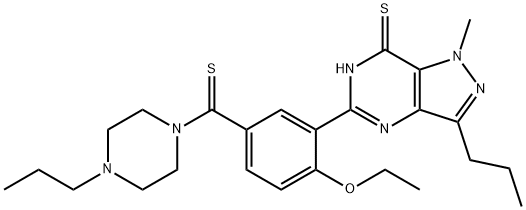
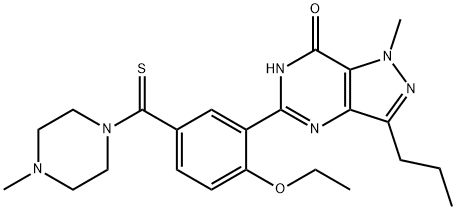

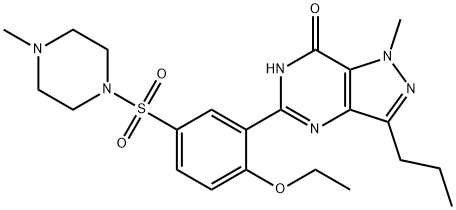
![4-ethoxy-3-(1-methyl-7-oxo-3-propyl-4H-pyrazolo[4,3-d]pyrimidin-5-yl)benzenesulfonamide](https://img.chemicalbook.in/CAS/20180719/GIF/372089-76-4.gif)
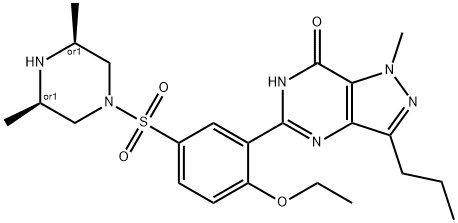
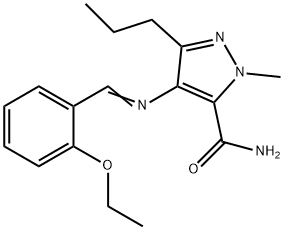
![1H-Pyrazolo[4,3-d]pyriMidine, 7-ethoxy-5-[2-ethoxy-5-[(4- Methyl-1-piperazinyl)sulfonyl] phenyl]-1-Methyl-3-propyl-](https://img.chemicalbook.in/CAS/20150408/GIF/1093065-15-6.gif)
You may like
-
 Sildenafil 98% (HPLC) CAS 139755-83-2View Details
Sildenafil 98% (HPLC) CAS 139755-83-2View Details
139755-83-2 -
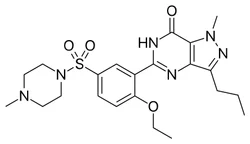 Sildenafil API PowderView Details
Sildenafil API PowderView Details
139755-83-2 -
 Sildenafil 100mg Eufile Tab, 10*1*4View Details
Sildenafil 100mg Eufile Tab, 10*1*4View Details
139755-83-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
