Tungsten hexafluoride
Synonym(s):Hexafluorotungsten;Tungsten hexafluoride
- CAS NO.:7783-82-6
- Empirical Formula: F6W
- Molecular Weight: 297.83
- MDL number: MFCD00040536
- EINECS: 232-029-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-31 14:42:41
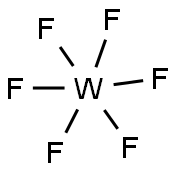
What is Tungsten hexafluoride?
Description
Tungsten(VI) fluoride, also known as tungsten hexafluoride, WF6, is a toxic, corrosive, colorless gas, with a density of about 13 g/L (roughly 11 times heavier than air). It is one of the densest gases known under standard conditions. WF6 is frequently used by the semiconductor industry to form tungsten films, through CVD. The WF6 molecule is octahedral with the symmetry point group of Oh. The W-F bond distances are 183.2 pm. Between 2.3℃ and 17℃, tungsten hexafluoride condenses into a pale-yellow liquid with a density of 3.44 g/cm3 at 15℃. At 2.3℃ it freezes into a white solid with a cubic crystalline structure, with lattice constant of 628 pm and a calculated density of 3.99 g/cm3 . At 29℃ this structure converts to an orthorhombic solid with lattice constants of a = 960.3 pm, b = 871.3 pm, and c = 504.4 pm and a density of 4.56 g/cm3 . In this phase, the W-F distance is 181 pm, and the mean closest intermolecular contacts are 312 pm.
Chemical properties
Colorless gas or light-yellow liquid.
Chemical properties
Tungsten hexafluoride is a toxic, colorless gas or a light yellow liquid.
The Uses of Tungsten hexafluoride
In the electronics industry as a source of tungsten metal that connects the aluminum layers within semiconductor devices.
General Description
A toxic corrosive light yellow liquid or gas. Boiling point 67°F. Melting point 37°F. Noncombustible. Used in the manufacture of other chemicals and in the manufacture of electronics.
Air & Water Reactions
Decomposes in water giving hydrofluoric acid, another corrosive material.
Reactivity Profile
Tungsten hexafluoride emits very toxic and irritating fumes containing metallic tungsten and tungsten fluorides when heated to decomposition. Reacts violently with tetramethoxysilane [Jacob, E., Angew. Chem., 1982, 21, p. 143].
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Vapors are extremely irritating and corrosive. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
A poison and corrosive liquid or gas.
Synthesis
WF6 is usually produced by the exothermic reaction of F2 gas with tungsten powder between 350℃ and 400℃.
W +3F2→WF6
Potential Exposure
A strong halogenating agent. Used to apply tungsten coatings to other surfaces by vapor deposition process; making electronics and components; in the manufacture of other chemicals.
Shipping
UN2196 Tungsten hexafluoride, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 8-Corrosive material, Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Incompatibilities
Decomposes on contact with water and moist air, forming highly corrosive hydrofluoric acid. Violent reaction on contact with methyl silicate.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Nonrefillable cylinders should be disposed of in accordance with local, state and federal regulations. Allow remaining gas to vent slowly into atmosphere in an unconfined area or exhaust hood. Refillabletype cylinders should be returned to original supplier with any valve caps and outlet plugs secured and valve protection caps in place.
Properties of Tungsten hexafluoride
| Melting point: | 2.3 °C(lit.) |
| Boiling point: | 17.5 °C(lit.) |
| Density | d15liq 3.441 |
| vapor pressure | 10hPa at 20℃ |
| solubility | reacts with H2O; very soluble in ctc,cyclohexane |
| form | colorless gas |
| color | yellow |
| Water Solubility | decomposes |
| Merck | 13,9885 |
| CAS DataBase Reference | 7783-82-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Tungsten hexafluoride(7783-82-6) |
| EPA Substance Registry System | Tungsten fluoride (WF6), (OC-6-11)- (7783-82-6) |
Safety information for Tungsten hexafluoride
| Signal word | Danger |
| Pictogram(s) |
 Gas Cylinder Compressed Gases GHS04  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H280:Gases under pressure H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tungsten hexafluoride
New Products
(R)-(-)-2-Amino-1-propanol Tetrahydro-4H-pyran-4-one Bromine 99.5% AR (4 x 500ml) Linseed Oil Extrapure Hydrogen Sulphide Solution Oxalic Acid 0.05 mol/L (0.01N) Solution EDTA 0.1 mol/L (0.2N) for 1000 ml Ferroin 0.025M Solution AR 1,2,3,4-Tetrahydrocarbazol-4-one 4-Hydroxy Carbazole Amino Salicylic Acid. U.S.P. 2 – Methoxy – 5- Sulfamoyl Benzoic acid Acetone Isobutryl oxime ester Sodium Amino Salicylate Dihydrate (PAS Sodium) IP/BP/USP/EP Aloe vera extract 200x Withania somnifera (Ashwagandha Extract) Citrus bioflavonoids Extract Spirulina (Arthrospira platensis) Powder Trikatu Extract Thymol Oil Ethyl 3-(Pyridin-2-Ylamino)Propanoate Bilastine -IP/BP/ Cypermethric Acid Chloride 5-NitrosalicylaldehydeRelated products of tetrahydrofuran


You may like
-
 7726-95-6 Bromine 99.5% AR (4 x 500ml) 99%View Details
7726-95-6 Bromine 99.5% AR (4 x 500ml) 99%View Details
7726-95-6 -
 Formamide 99%View Details
Formamide 99%View Details
75-12-7 -
 2, 4-Pyrimidinediamine 3-Oxide 99%View Details
2, 4-Pyrimidinediamine 3-Oxide 99%View Details
74638-76-9 -
 85-81-4 6-Methoxy-8-Nitroquinoline 99%View Details
85-81-4 6-Methoxy-8-Nitroquinoline 99%View Details
85-81-4 -
![3-Bromo-4,5-Dihydro-1H-Benzo[B]Azepin-2(3H)-One 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 3-Bromo-4,5-Dihydro-1H-Benzo[B]Azepin-2(3H)-One 99%View Details
3-Bromo-4,5-Dihydro-1H-Benzo[B]Azepin-2(3H)-One 99%View Details
86499-96-9 -
 (−)-Dip-Chloride 85116-37-6 99%View Details
(−)-Dip-Chloride 85116-37-6 99%View Details
85116-37-6 -
 29943-42-8 Tetrahydro-4H-pyran-4-one 98+View Details
29943-42-8 Tetrahydro-4H-pyran-4-one 98+View Details
29943-42-8 -
 35320-23-1 98+View Details
35320-23-1 98+View Details
35320-23-1