Tryptophol
Synonym(s):2-(3-Indolyl)ethanol;3-Indoleethanol;IEA;NSC 3884;Tryptophol
- CAS NO.:526-55-6
- Empirical Formula: C10H11NO
- Molecular Weight: 161.2
- MDL number: MFCD00005659
- EINECS: 208-393-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
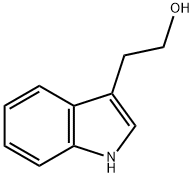
What is Tryptophol?
Chemical properties
3-(2-Hydroxyethyl)indole [526-55-6], tryptophol, C10H11NO, Mr 161.20, mp 59 ℃, bp 174 ℃, (2 kPa) forms colorless prisms or flakes that are readily soluble in methanol, ethanol, diethyl ether, chloroform, and acetone, soluble in benzene, and slightly soluble in water. It is produced by reacting methyl-1H-indole-3-acetate with lithium aluminum hydride, or through reduction of methyl-1H-indole-3-glyoxylate with sodium borohydride. The antihypertensive Indoramin is produced from tryptophol.
The Uses of Tryptophol
Tryptophol is a metabolite formed in the liver after disulfiram treatment that induces sleep in humans. It is also a secondary product of alcoholic fermentation.
The Uses of Tryptophol
Reactant for preparation of:
- Inhibitors of the C-terminal domain of RNA polymerase II and their antitumor activities
- Anti-HIV-1 agents
- Inhibitors of Protein-Protein Interactions
- Partial agonists of the serotonin 5-HT1A receptor
- Growth hormone secretagogues
- Vascular endothelial growth factor (VEGF) inhibitors
- A2B adenosine receptor ligands
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- Inhibitors of interleukine 6
- Dual binding site acetylcholinesterase inhibitors
What are the applications of Application
3-(2-Hydroxyethyl)indole is an indole for the production of compounds used in biological research
Definition
ChEBI: An indolyl alcohol that is ethanol substituted by a 1H-indol-3-yl group at position 2.
Synthesis Reference(s)
Canadian Journal of Chemistry, 42, p. 485, 1964 DOI: 10.1139/v64-070
Purification Methods
Crystallise it from diethyl ether/pet ether, *C6H6, *C6H6/pet ether. The picrate has m 100-101o (from *C6H6). [Beilstein 21 I 218, 21 II 49, 21 III/IV 788, 21/3 V 61.]
Properties of Tryptophol
| Melting point: | 56-59 °C(lit.) |
| Boiling point: | 174 °C (2.02527 mmHg) |
| Density | 1.0840 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| Flash point: | 255 °C |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | methanol: 0.1 g/mL, clear |
| form | Crystalline Mass, Crystals, Flakes or Powder |
| pka | 15.16±0.10(Predicted) |
| color | Off-white to brown |
| Water Solubility | 10 g/L (20 ºC) |
| Merck | 14,9798 |
| BRN | 125553 |
| CAS DataBase Reference | 526-55-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-indole-3-ethanol(526-55-6) |
| EPA Substance Registry System | 1H-Indole-3-ethanol (526-55-6) |
Safety information for Tryptophol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Tryptophol
| InChIKey | MBBOMCVGYCRMEA-UHFFFAOYSA-N |
Tryptophol manufacturer
A.J Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Tryptophol 98% CAS 526-55-6View Details
Tryptophol 98% CAS 526-55-6View Details
526-55-6 -
 3-Indoleethanol CAS 526-55-6View Details
3-Indoleethanol CAS 526-55-6View Details
526-55-6 -
 Tryptophol 99% (HPLC) CAS 526-55-6View Details
Tryptophol 99% (HPLC) CAS 526-55-6View Details
526-55-6 -
 3-(2-Hydroxyethyl)indole CAS 526-55-6View Details
3-(2-Hydroxyethyl)indole CAS 526-55-6View Details
526-55-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
