Tryptamine
Synonym(s):2-(3-Indolyl)ethylamine;3-(2-Aminoethyl)indole
- CAS NO.:61-54-1
- Empirical Formula: C10H12N2
- Molecular Weight: 160.22
- MDL number: MFCD00005661
- EINECS: 692-120-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
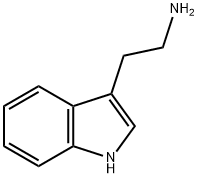
What is Tryptamine?
Description
Tryptamine is an indole alkaloid and intermediate in the biosynthesis of serotonin and the phytohormone melatonin in plants. It increases the levels of the terpenoid indole alkaloids ajmalicine, strictosidine, and catharanthine in cultures of C. roseus. Tryptamine is also a product of tryptophan metabolism in mammals. Tryptamine derivatives have been synthetically produced as hallucinogenic drugs of abuse that act on the serotonergic system.
Chemical properties
Tryptamine is a biogenic imine derived from the decarboxylation of tryptophan. It is a white to orange crystalline Powder, melting point 118°C (decomposition at 145-146°C). Soluble in ethanol and acetone, almost insoluble in ether, benzene, chloroform and water.
The Uses of Tryptamine
Tryptamine is a monoamine alkaloid found in plants. Tryptamine is commonly used in the preparation of biologically active compounds such as neurotransmitters and psychedelics.
What are the applications of Application
Tryptamine is a vasoactive, biogenic amine compound which may function as a neuromodulator
Definition
ChEBI: Tryptamine is an aminoalkylindole consisting of indole having a 2-aminoethyl group at the 3-position. It has a role as a human metabolite, a plant metabolite and a mouse metabolite. It is an aminoalkylindole, an indole alkaloid, an aralkylamino compound and a member of tryptamines. It is a conjugate base of a tryptaminium.
Preparation
Tryptamine, a monoamine alkaloid containing an indole ring structure is derived by the decarboxylation of amino acid tryptophan.
The synthesis of tryptamines is typically conducted following a classic route starting with a Mannich reaction of an indole heterocycle, followed by quaternization of the amine, nucleophilic substitution with highly toxic cyanide and final reduction.
General Description
Tryptamines which are usually found in plants, fungi, animals, etc. are categorized under the monoamine alkaloids class of compounds.
Biochem/physiol Actions
Vasoactive; may have a neuromodulator function; biogenic amine formed from the decarboxylation of tryptophan by L-aromatic amino acid decarboxylase.
Purification Methods
Crystallise tryptamine from *benzene, Et2O (m 114o) or pet ether (m 118o). It has UV: 222n 276, 282 and 291nm (EtOH) and max 226, 275, 281 and 290nm (HCl). [Beilstein 22 II 346, 22 III/IV 4319, 22/10 V 45.]
Properties of Tryptamine
| Melting point: | 113-116 °C (lit.) |
| Boiling point: | 137 °C/0.15 mmHg (lit.) |
| Density | 0.9787 (rough estimate) |
| refractive index | 1.6210 (estimate) |
| Flash point: | 185 °C |
| storage temp. | 2-8°C |
| solubility | water: soluble1g/L at 20°C |
| form | crystalline |
| pka | 10.2(at 25℃) |
| color | white |
| PH | 11.07 (10g/l, H2O, 24.7℃) |
| Water Solubility | negligible |
| Sensitive | Air Sensitive |
| Merck | 14,9796 |
| BRN | 125513 |
| CAS DataBase Reference | 61-54-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-Indole-3-ethanamine(61-54-1) |
| EPA Substance Registry System | Tryptamine (61-54-1) |
Safety information for Tryptamine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tryptamine
Tryptamine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Tryptamine 99% supplierView Details
Tryptamine 99% supplierView Details
61-54-1 -
 61-54-1 Tryptamine, 99% 99%View Details
61-54-1 Tryptamine, 99% 99%View Details
61-54-1 -
 Tryptamine, 99% CAS 61-54-1View Details
Tryptamine, 99% CAS 61-54-1View Details
61-54-1 -
 Tryptamine CAS 61-54-1View Details
Tryptamine CAS 61-54-1View Details
61-54-1 -
 Tryptamine 99% CAS 61-54-1View Details
Tryptamine 99% CAS 61-54-1View Details
61-54-1 -
 Tryptamine CAS 61-54-1View Details
Tryptamine CAS 61-54-1View Details
61-54-1 -
 Tryptamine CAS 61-54-1View Details
Tryptamine CAS 61-54-1View Details
61-54-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
