2-(2-Aminoethylamino)ethanol
Synonym(s):N-(2-Aminoethyl)ethanolamine;2-(2-Aminoethylamino)ethanol
- CAS NO.:111-41-1
- Empirical Formula: C4H12N2O
- Molecular Weight: 104.15
- MDL number: MFCD00008170
- EINECS: 203-867-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
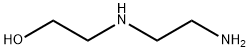
What is 2-(2-Aminoethylamino)ethanol?
Description
Aminoethylethanolamine is a component of colophony in soldering flux, which may cause contact and airborne contact dermatitis in workers in the electronic industry or in cable jointers.
Chemical properties
CLEAR VISCOUS LIQUID
Chemical properties
Aminoethylethanolamine is combustible, colorless, liquid with an ammonia-like odor.
The Uses of 2-(2-Aminoethylamino)ethanol
Textile finishing compounds (antifuming agents, dyestuffs, cationic surfactants), resins, rubber products, insecticides, and certain medicinals.
The Uses of 2-(2-Aminoethylamino)ethanol
It is used as an important intermediate in the manufacture of lube oil additives, fuel additives, chelating agents, surfactants and fabric softeners among other applications. N-(2-Hydroxyethyl)ethylenediamine [N-(2-Aminoethyl)ethanolamine] is used to study the aerobic biodecomposition of amines in hypersaline wastewaters.
The Uses of 2-(2-Aminoethylamino)ethanol
N-(2-hydroxyethyl)ethylenediamine (hydeten) or [N-(2-aminoethyl)ethanolamine] can be used as:
- A precursor in the synthesis of room-temperature ionic liquids, acetate and formate ammonium salts of N-(2-hydroxyethyl)-ethylenediamine.
- A ligand in the preparation of polymeric cyano-bridged platinum(II)complexes, ligand-copper(II)carbohydrate complexes and cyano-bridged Ni-Pt and Cu-Pt coordination polymers.
It can also be used to study the aerobic biodecomposition of amines in hypersaline wastewaters.
General Description
A clear colorless liquid with an ammonia-like odor. Corrosive to tissue. Combustible, but may be difficult to ignite. Less dense than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion. Used to make other chemicals.
Air & Water Reactions
Water soluble. Hygroscopic.
Reactivity Profile
2-(2-Aminoethylamino)ethanol is an amine and alcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. 2-(2-Aminoethylamino)ethanol is hygroscopic.
Health Hazard
Skin contact will cause mild irritation; eye contact will cause more severe irritation.
Fire Hazard
2-(2-Aminoethylamino)ethanol is combustible.
Contact allergens
Aminoethylethanolamine is a component of colophony in soldering flux, which may cause contact and airborne contact dermatitis in workers in the electronic industry or cable jointers.
Potential Exposure
Used to make textile finishing compounds, dyes, resins, rubber, insecticides, medicines, and oth
Shipping
UN2735 Amines, liquid, corrosive, n.o.s, or Polyamines, liquid, corrosive, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.
Purification Methods
Distil the amine twice through a Vigreux column (p 11). Redistil it from solid NaOH, then from CaH2. Alternatively, it can be converted to the dihydrochloride and recrystallised from water. It is then dried, mixed with excess of solid NaOH and the free base is distilled from the mixture. It is finally redistilled from CaH2. [Drinkard et al. J Am Chem Soc 82 2992 1960, Beilstein 4 IV 1558.]
Incompatibilities
Contact with cellulose nitrate may cause fires upon contact. Reacts with Oxidizers, strong acids.
Properties of 2-(2-Aminoethylamino)ethanol
| Melting point: | -28 °C |
| Boiling point: | 238-240 °C/752 mmHg (lit.) |
| Density | 1.03 g/mL at 25 °C (lit.) |
| vapor density | 3.6 (vs air) |
| vapor pressure | <0.01 hPa (20 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | soluble |
| form | Liquid |
| pka | pK1:7.21(+2);pK2:10.12(+1) (25°C) |
| color | Clear Colorless |
| PH | 11.8 (111g/l, H2O, 20℃) |
| Odor | mild ammoniacal odor |
| explosive limit | 3.3-10.1%(V) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| BRN | 506012 |
| Dielectric constant | 18.989999999999998 |
| Exposure limits | NIOSH: TWA 0.1 mg/m3 |
| CAS DataBase Reference | 111-41-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethanol, 2-[(2-aminoethyl)amino]-(111-41-1) |
| EPA Substance Registry System | N-Hydroxyethylethylenediamine (111-41-1) |
Safety information for 2-(2-Aminoethylamino)ethanol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-(2-Aminoethylamino)ethanol
| InChIKey | LHIJANUOQQMGNT-UHFFFAOYSA-N |
2-(2-Aminoethylamino)ethanol manufacturer
Multichem Specialities Pvt. Ltd.
Paragon Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 AMINO ETHYL ETHANOLAMINE 99%View Details
AMINO ETHYL ETHANOLAMINE 99%View Details -
 Amino Ethyl Ethanolamine CASView Details
Amino Ethyl Ethanolamine CASView Details -
 Amino Ethyl Ethanolamine CASView Details
Amino Ethyl Ethanolamine CASView Details -
 Amino Ethyl EthanolamineView Details
Amino Ethyl EthanolamineView Details
111-41-1 -
 Aminoethyl EthanolamineView Details
Aminoethyl EthanolamineView Details
111-41-1 -
 N-(2-Aminoethyl) EthanolamineView Details
N-(2-Aminoethyl) EthanolamineView Details
111-41-1 -
 Amino Ethyl Ethanolamine, For Industrial, Grade: Technical GradeView Details
Amino Ethyl Ethanolamine, For Industrial, Grade: Technical GradeView Details
111-41-1 -
 Liquid N-(2-Aminoethyl) Ethanolamine, for Commerical, Packaging Type: Plastic DrumView Details
Liquid N-(2-Aminoethyl) Ethanolamine, for Commerical, Packaging Type: Plastic DrumView Details
111-41-1
