Troxerutin
Synonym(s):3′,4′,7-Tris[O-(2-hydroxyethyl)]rutin;Trihydroxyethylrutin;Troxerutin
- CAS NO.:7085-55-4
- Empirical Formula: C33H42O19
- Molecular Weight: 742.68
- MDL number: MFCD00893813
- EINECS: 230-389-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-29 14:06:09
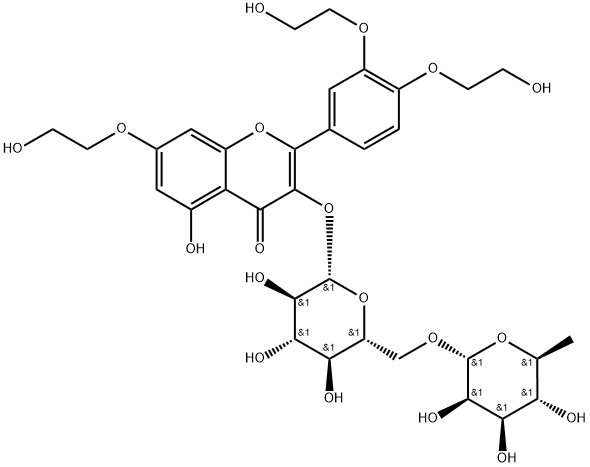
What is Troxerutin?
Chemical properties
Yellow Powder
Originator
Venoruton,Novartis Co
The Uses of Troxerutin
Used in the treatment of venous disorders
The Uses of Troxerutin
vasoprotectant
The Uses of Troxerutin
Troxerutin, a derivative of the naturally occurring bioflavonoid rutin, is thought to inhibit red cell and platelet aggregation, improve erythrocyte deformability, and improve plasma viscosity retinal microcirculation. A double-blind randomized clinical trial compared troxerutin with placebo in 27 patients with CRVO. The limitations of the study included a small number of patients and short follow-up time.
What are the applications of Application
Trihydroxyethylrutin is a flavonoid derivative which is effective in protecting membranes from oxidative DNA damage.
Manufacturing Process
In a nitrogen atmosphere 120 g of caustic soda (3 moles) in solution are added to 610 g of rutin (1 mol) suspended in 2 litres of water, the mixture being vigorously agitated by a mechanical stirrer, 241.5 g of ethylene chlorohydrin being then introduced at 55°C for 10 min. When all the chlorohydrin has been thus added the temperature is progressively raised to 75°C and maintained at this level for 2 hours. After cooling, in a nitrogen atmosphere, the pH value adjusted to 5 by the addition of dilute hydrochloric acid. The solution is kept in an ice box for 24 hours and then filtered to remove any impurities. At reduced pressure the solution is evaporated until dry, the residue taken up in 3 litres of boning methanol which dissolves the tri-(β-hydroxyethyl)rutin formed and leaves the sparingly soluble sodium chloride behind. The tri-(β-hydroxyethyl)rutin is recovered from its methanolic solution either by evaporation and refrigeration, or by evaporation precipitation with absolute ethanol. In either case tri-(β-hydroxyethyl)rutin obtained is in the form of small very hygroscopic crystals which contain alcohol of crystallization.These crystals are quickly shaken washed in a little cold absolute ethanol and then dried in vacuum at 100°C. 680 g of anhydrous tri-(β-hydroxyethyl)rutin are thus obtained in the form of a yellow powder which melts at 156°C.GB Patent No. 833,174; April 21, 1960; Assigned to Zyma S.A., a Swiss Corporation, of Route Etraz, Nyon Canton of Vaud, Switzerland
Therapeutic Function
Topical venotonic
Properties of Troxerutin
| Melting point: | 181°C |
| Boiling point: | 1058.4±65.0 °C(Predicted) |
| Density | 1.65±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Freely soluble in water, slightly soluble in ethanol (96 per cent) and practically insoluble in methylene chloride. |
| form | neat |
| pka | 5.92±0.40(Predicted) |
| form | Solid |
| color | Light Yellow to Yellow |
| Merck | 14,9789 |
| BRN | 4778232 |
| CAS DataBase Reference | 7085-55-4(CAS DataBase Reference) |
Safety information for Troxerutin
Computed Descriptors for Troxerutin
| InChIKey | KMPBUGGPUQOWMJ-NXPSPVSKSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 7085-55-4 Troxerutin 99%View Details
7085-55-4 Troxerutin 99%View Details
7085-55-4 -
 7085-55-4 98%View Details
7085-55-4 98%View Details
7085-55-4 -
 Troxerutin 98%View Details
Troxerutin 98%View Details
7085-55-4 -
 Troxerutin CAS 7085-55-4View Details
Troxerutin CAS 7085-55-4View Details
7085-55-4 -
 Troxerutin, 95% CAS 7085-55-4View Details
Troxerutin, 95% CAS 7085-55-4View Details
7085-55-4 -
 Troxerutin 95% (HPLC) CAS 7085-55-4View Details
Troxerutin 95% (HPLC) CAS 7085-55-4View Details
7085-55-4 -
 Troxerutin >90% CAS 7085-55-4View Details
Troxerutin >90% CAS 7085-55-4View Details
7085-55-4 -
 Troxerutin CAS 7085-55-4View Details
Troxerutin CAS 7085-55-4View Details
7085-55-4
