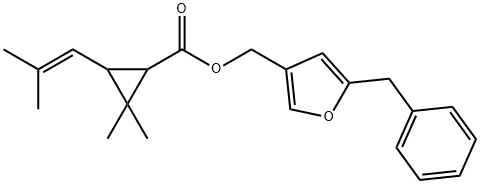
RESMETHRIN
- CAS NO.:10453-86-8
- Empirical Formula: C22H26O3
- Molecular Weight: 338.44
- MDL number: MFCD00041806
- EINECS: 233-940-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:40
What is RESMETHRIN?
Chemical properties
Off-white to tan waxy solid or colorless crys- tals. Chrysanthemum-like odor. Commercial products may be dissolved in flammable organic solvents
The Uses of RESMETHRIN
Resmethrin is used to control a wide range of insects in horticultural, household, public health and animal health situations. It has some agricultural use but this is limited. The more active 1Rtrans form has a similar range of uses and is also used in stored grain products.
What are the applications of Application
Resmethrin is a neural Ca2+-calmodulin-dependent protein phosphatase inhibitor
Definition
ChEBI: Resmethrin is a member of furans and a cyclopropanecarboxylate ester. It has a role as a pyrethroid ester insecticide and an agrochemical. It is functionally related to a chrysanthemic acid.
General Description
Colorless crystals or waxy solid. Insoluble in water. Used as an insecticide.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A pyrethroid derivative.
Agricultural Uses
Insecticide: Resmethrin is currently used for mosquito control (by aerial application) in the USA, and may can also be used in greenhoused to control white fly. Resmethrin is a synthetic Type I pyrethroid insecticide registered for control of insects in residential, commercial and industrial settings, and in animal living areas. Resmethrin is also registered for use in food-handling establishments and as a restricted use pesticide when used in ULV spray to control adult mosquitoes in the interest of public health[83]. Restricted Use Pesticide (RUP) when formulated for use in mosquito abatement and pest control treatments at nonagricultural sites. Restricted due to extreme fish toxicity. Not approved for use in EU countries.
Trade name
(d-trans-isomer); SBP® 1382 (d-trans-isomer); d-transSBP® 1382 (d-trans-isomer); SBP®-1390; S. B. PENICK 1382®; SCOURGE®; SUN-BUGGER®; SYNTHRIN®; SYNTOX®; VECTRIN®; WHITMIRE® PT-110
Safety Profile
Poison by ingestion, andintravenous routes. Moderately toxic by inhalation andskin contact. When heated to decomposition it emits acridand irritating fumes.
Potential Exposure
Resmethrin is pyrethroid insecticide used for mosquito control (by aerial application) in the USA, and may can also be used in greenhouses to control white fly. Resmethrin is a synthetic Type I pyrethroid insecticide registered for control of insects in residential, commercial, and industrial settings, and in animal living areas. It is also registered for use in food handling estab- lishments and as a restricted use pesticide when used in ULV spray to control adult mosquitoes in the interest of public health . A United States Restricted Use Pesticide (RUP) when formulated for use in mosquito abatement and pest control treatments at nonagricultural sites. Restricted due to extreme fish toxicity.
Metabolic pathway
14C-resmethrin are individually administered orally to white leghorn hens, and more than 90% of the radioactivity is eliminated in the excreta within 24 h of treatment. The metabolic routes for both resmethrin isomers arise from ester hydrolysis and oxidation of the hydrolytic products. Some of these metabolites are further conjugated with glucuronic acid, sulfate, or other unidentified compounds before excretion.
Shipping
UN3352 Pyrethroid pesticide, liquid toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3349 Pyrethroid pesticide, solid toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous material
Degradation
The resmethrins are reasonably stable but they are sensitive to base
hydrolysis forming the chrysanthemic acid isomers (2) and 5-benzyl-3-
furylmethanol (3). They are also sensitive to oxidation and to photodecomposition.
Photo-oxidation involves degradation of the furan ring
to yield a cyclic ozonide peroxide by addition of oxygen across the unsaturated
system forming the hydroxylactone derivative (4), the benzyloxylactone
derivative (5) and the hydroxycyclopentenolone (6) (Ueda
et al., 1974). These chemical and photochemical reactions are shown in
Scheme 1.
Degradation also occurs in the acid moiety by reactions analogous to
those described under phenothrin, for example, oxidation at the isobutylene
substituent to afford a variety of alcohols, aldehydes and carboxylic acids. These two sites of weakness in the resmethrin molecule result
in considerable photo-instability (though they are more stable than the
pyrethrins) and to a complex mixture of degradation products.
Incompatibilities
Decomposed by air, light, alkaline media, and temperatures .175℃. May react violently with strong oxidizers, bromine, 90% hydrogen peroxide, phos- phorus trichloride, silver powders or dust. Incompatible with silver compounds. Mixture with some silver compounds forms explosive salts of silver oxalate.
Waste Disposal
Incineration would be an effective disposal procedure where permitted. If an efficient incinerator is not available, the product should be mixed with large amounts of combustible material and contact with the smoke should be avoided. In accordance with 40CFR165. Follow recommendations for the disposal of pesticides and pesticide containers . Bury in noncrop land away from water. It would be better to mix the prod- uct with lime. Incineration would be an effective disposal procedure where permitted. If an efficient incinerator is not available, the product should be mixed with large amount of combustible material. Recommendable methods: Hydrolysis, landfill, incineration, and open burning. Not recommendable method: Discharge to sewer. Mix with saw- dust and burn at a remote place .
Properties of RESMETHRIN
| Melting point: | 56.5℃ |
| Boiling point: | bp >180° (dec) |
| Density | 0.96 g/cm3 |
| vapor pressure | ﹤l×10-5 Pa (25 °C) |
| refractive index | 1.5287 (20℃) |
| Flash point: | closed cup: 264.2°F (129°C) |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly, Heated) |
| form | neat |
| Water Solubility | 0.038 mg l-1 (25 °C) |
| BRN | 1626510 |
| EPA Substance Registry System | Resmethrin (10453-86-8) |
Safety information for RESMETHRIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for RESMETHRIN
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Resmethrin CAS 10453-86-8View Details
Resmethrin CAS 10453-86-8View Details
10453-86-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
