Triphosgene
Synonym(s):Bis(trichloromethyl) carbonate;Triphosgene;tri-Phosgene;Triphosgene, Carbonic acid bistrichlor methyl ester
- CAS NO.:32315-10-9
- Empirical Formula: C3Cl6O3
- Molecular Weight: 296.73
- MDL number: MFCD00062848
- EINECS: 250-986-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
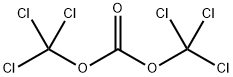
What is Triphosgene?
Description
Triphosgene is also known as solid phosgene. Its chemical name is bis (Trichloromethyl) carbonate, and its English name is bisgriehloromethyl) carbonate or triphosgene, abbreviated as BTC. Triphosgene is a white crystal, similar to the smell of phosgene. It is mainly used to synthesize chloroformate, isocyanate, polycarbonate and acyl chloride. It is widely used as an intermediate in plastics, medicine, herbicides and pesticides.
Chemical properties
White solid
The Uses of Triphosgene
Triphosgene is used as a carbonylating agent for aza-peptide synthesis. It reacts with several alfa-amino acids to give the corresponding N-carboxyanhydrides. It is involved in the preparation of the esterification coupling reagent, di-2-thienyl carbonate from 2(5H)-thiophenone. Further, it is used as a reagent in organic synthesis and converts an amino group into isocyanate. In addition to this, it is employed in the preparation of 2-chloronicotinaldehydes through cyclization of the corresponding enamides. It is considered as a useful substitute for phosgene.
The Uses of Triphosgene

To a solution of triphosgene (26.4 mg, 0.090 mmol) in ACN (1 mL) at -5 C was added a solution of B (91.6 mg, 0.26 mmol) and TEA (73.2 uL) in ACN (1 mL) over 5 min. Then was added a solution of A (125 mg, 0.090 mmol) and TEA (73.2 uL) in ACN (1 mL). The reaction mixture was slowly warmed to RT and stirred for 3 h. The mixture was concentrated and the resulting material was purified by RP-HPLC to provide the product as a flaky shiny white solid. [132 mg, 75%]
Reactions
The Triphosgene reaction is quenched very carefully by dropwise addition of 75 mL of saturated aqueous sodium sulfate solution (Notes 5 and 6). The resulting white granular precipitate or slurry is removed by vacuum filtration through Celite, and the filter cake is washed with three portions of 75 mL of chloroform .
General Description
Triphosgene, also known as bis(trichloromethyl) carbonate or BTC, is a chemical compound used in organic synthesis as a reagent for several types of chemical transformations. As a highly effective reagent, it serves as a carbonylating agent, making it invaluable in the synthesis of isocyanates and other carbonyl compounds.
Safety
Triphosgene's low vapor pressure makes it possible for it to reach concentrations that are considered toxicologically unsafe. While several properties of triphosgene are not yet readily available, it is known that it is extremely toxic if inhaled. A toxic gas is emitted if it comes in contact with water. There is a lack of information and variability regarding the proper handling of triphosgene. It is assumed to have the same risks as phosgene.
Synthesis
Triphosgene is synthesized by exhaustive free radical chlorination of dimethyl carbonate:
CH3OCO2CH3 + 6 Cl2 → CCl3OCO2CCl3 + 6 HCl
Triphosgene can be easily recrystallized from hot hexanes.
Purification Methods
It is a good solid substitute for phosgene (using a third mol per mol). Crystallise it from pet ether (b 60-80o), wash it with anhydrous cold Et2O, de-gas it at 200mm then dry at 0.1mm (over H2SO4). It has IR: max 900 and 1900 cm-1 . It is a lachrymator, is TOXIC and should be handled with gloves and in an efficient fume hood. [Hales et al. J Chem Soc 620 1957, Eckert & Forster Angew Chem, Int Ed Engl 26 894 1987, Aldrichimica Acta 21 47 1988, Beilstein 3 H 17, 3 I 8, 3 II 16, 3 III 36, 3 IV 33.]
Properties of Triphosgene
| Melting point: | 79-83 °C (lit.) |
| Boiling point: | 203-206 °C (lit.) |
| Density | 1.78 |
| vapor pressure | 16 hPa (90 °C) |
| Flash point: | 203-206°C |
| storage temp. | 2-8°C |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| appearance | White solid |
| form | Crystalline Powder, Crystals and/or Chunks |
| color | White to off-white |
| Water Solubility | practically insoluble |
| Sensitive | Moisture Sensitive |
| BRN | 1787583 |
| CAS DataBase Reference | 32315-10-9(CAS DataBase Reference) |
| EPA Substance Registry System | Methanol, trichloro-, carbonate (2:1) (32315-10-9) |
Safety information for Triphosgene
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Triphosgene
| InChIKey | UCPYLLCMEDAXFR-UHFFFAOYSA-N |
Triphosgene manufacturer
JSK Chemicals
Leo Chemo Plast Pvt. Ltd.
Multichem Specialities Pvt. Ltd.
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
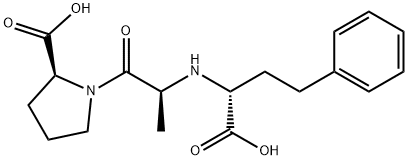
![D-Proline, N-[(1S)-1-carboxy-3-phenylpropyl]-L-alanyl-](https://img.chemicalbook.in/CAS/20180527/GIF/196083-21-3.gif)
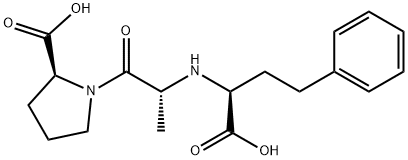
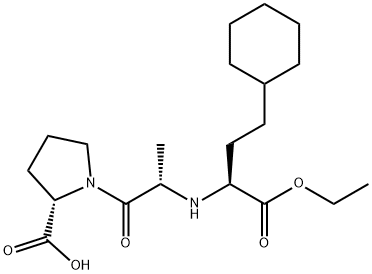
![(S)-1-[N-[3-Phenyl-1-[(phenylMethoxy)carbonyl]propyl]-L-alanyl]-L-proline](https://img.chemicalbook.in/CAS/GIF/76391-33-8.gif)
You may like
-
 TRIPHOSGENE 99%View Details
TRIPHOSGENE 99%View Details -
 Triphosgene 98%View Details
Triphosgene 98%View Details -
 Triphosgene 99%View Details
Triphosgene 99%View Details -
 Triphosgene CASView Details
Triphosgene CASView Details -
 Triphosgene CASView Details
Triphosgene CASView Details -
 Triphosgene CASView Details
Triphosgene CASView Details -
 TRIPHOSGENE, Grade: Industrial, Purity: 99 %View Details
TRIPHOSGENE, Grade: Industrial, Purity: 99 %View Details
32315-10-9 -
 Powder Triphosgene, Packaging Type: Drum ,Grade Standard: Reagent GradeView Details
Powder Triphosgene, Packaging Type: Drum ,Grade Standard: Reagent GradeView Details
32315-10-9
