Triphenylmethane
Synonym(s):Triphenylmethane;Tritan
- CAS NO.:519-73-3
- Empirical Formula: C19H16
- Molecular Weight: 244.33
- MDL number: MFCD00004763
- EINECS: 208-275-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
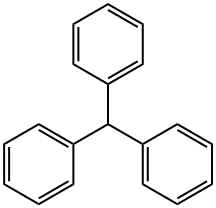
What is Triphenylmethane?
Chemical properties
colorless or light brown powder. soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical).
The Uses of Triphenylmethane
Triphenylmethane is a triarylmethane compound used as the backbone of synthetic dyes. Triphenylmethane has also been shown to inhibit 3-methylcholanthrene-induced neoplastic transformation of 10T1/2 cells.
Definition
ChEBI: Triphenylmethane is a triarylmethane in which the three aryl groups are phenyl. It forms the basic skeleton of several synthetic dyes. It has a role as a xenobiotic and an environmental contaminant.
Preparation
Triphenylmethane can be synthesized by Friedel–Crafts reaction from benzene and chloroform with aluminium chloride catalyst:
3 C6H6 + CHCl3 → Ph3CH + 3 HCl
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 5846, 1951 DOI: 10.1021/ja01156a116
Organic Syntheses, Coll. Vol. 1, p. 548, 1941
Tetrahedron Letters, 21, p. 801, 1980 DOI: 10.1016/S0040-4039(00)71509-7
Purification Methods
Crystallise triphenylmethane from EtOH or *benzene (with one molecule of *benzene of crystallisation which is lost on exposure to air or by heating on a water bath). It can also be sublimed under vacuum. It has been given a preliminary purification by refluxing with tin and glacial acetic acid, then filtered hot through a glass sinter disc, and precipitated by addition of cold water. [Beilstein 5 H 698, 5 IV 2495.]
Properties of Triphenylmethane
| Melting point: | 92-94 °C(lit.) |
| Boiling point: | 358-359 °C754 mm Hg(lit.) |
| Density | 1.014 g/mL at 25 °C(lit.) |
| refractive index | nD100 1.59546 |
| Flash point: | 358-359°C |
| storage temp. | Store below +30°C. |
| solubility | dioxane: 0.1 g/mL, clear |
| form | Powder |
| pka | 31.5(at 25℃) |
| color | Faint yellow |
| Specific Gravity | 1.014 |
| Water Solubility | INSOLUBLE |
| Merck | 14,9741 |
| BRN | 1909753 |
| Dielectric constant | 3.6(65℃) |
| Stability: | Stable; combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 519-73-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Triphenylmethane(519-73-3) |
| EPA Substance Registry System | Benzene, 1,1',1''-methylidynetris- (519-73-3) |
Safety information for Triphenylmethane
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Triphenylmethane
Triphenylmethane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 519-73-3 TRIPHENYLMETHANE 98%View Details
519-73-3 TRIPHENYLMETHANE 98%View Details
519-73-3 -
 519-73-3 99%View Details
519-73-3 99%View Details
519-73-3 -
 519-73-3 99%View Details
519-73-3 99%View Details
519-73-3 -
 Triphenylmethane CAS 519-73-3View Details
Triphenylmethane CAS 519-73-3View Details
519-73-3 -
 Triphenylmethane 99% (GC) CAS 519-73-3View Details
Triphenylmethane 99% (GC) CAS 519-73-3View Details
519-73-3 -
 Triphenylmethane CAS 519-73-3View Details
Triphenylmethane CAS 519-73-3View Details
519-73-3 -
 Triphenylmethane CAS 519-73-3View Details
Triphenylmethane CAS 519-73-3View Details
519-73-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
