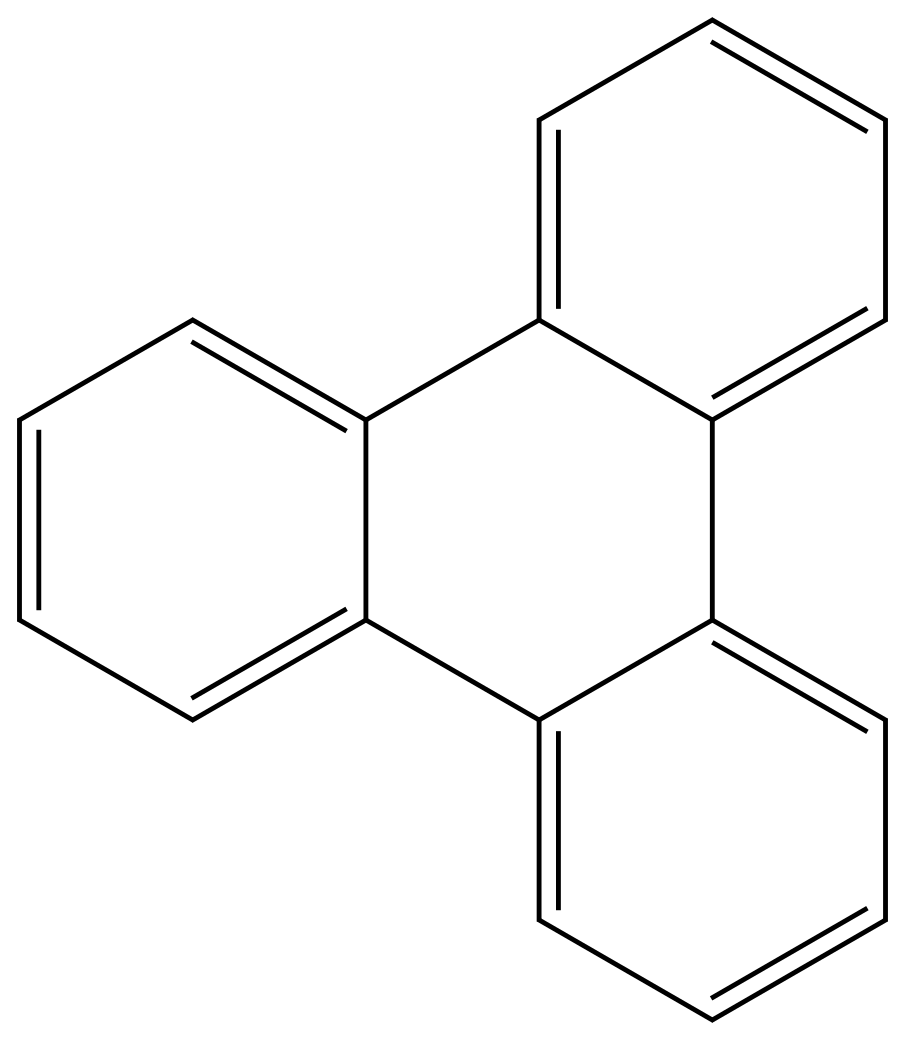
Triphenylene
Synonym(s):9,10-Benzophenanthrene
- CAS NO.:217-59-4
- Empirical Formula: C18H12
- Molecular Weight: 228.29
- MDL number: MFCD00001108
- EINECS: 205-922-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
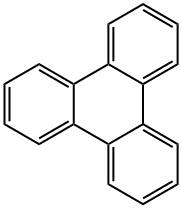
What is Triphenylene?
Chemical properties
white to beige crystalline needles
The Uses of Triphenylene
Triphenylene is a polycyclic aromatic hydrocarbon (PAH) that can be isolated from coal tar. Triphenylene emits fluorescence in the ultraviolet region and is a useful compound for developing semiconductor devices.
What are the applications of Application
Triphenylene is a compound that fluoresces in the ultraviolet region
Definition
ChEBI: Triphenylene is an ortho-fused polycyclic arene consisting of four fused benzene rings.
What are the applications of Application
Triphenylene is an important basic skeleton monomer of polycyclic aromatic hydrocarbons. It can be used to synthesize macromolecular compounds with multi-conjugated structure, and is widely used in the research of organic supramolecular materials.Triphenylene is used in optics and electronics. It is also used as a discotic mesogen in liquid crystalline materials. Further, it is a compound that fluoresces in the ultraviolet region. In addition to this, it is used in the preparation of triphenylene-2-carbaldehyde.
Preparation
Triphenylene synthesis: 2-Chlorobenzoic acid(78mg, 0.5mmol), iodonium salt (236mg, 0.55mmol), palladium acetate (2.8mg, 0.0125mmol), potassium carbonate (152mg, 1.1mmol) were dissolved in 3mL of NMP solvent was heated to 110 °C and stirred for 17 hours, cooled to room temperature, extracted with ethyl acetate, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, concentrated, and subjected to column chromatography to obtain 92 mg of triphenylene as a white solid, The yield was 80%.
Synthesis Reference(s)
Synthesis, p. 756, 1992 DOI: 10.1055/s-1992-26218
General Description
Triphenylene belongs to the class of polycyclic aromatic hydrocarbons, which is commonly employed as a substrate for use as a precursor for the synthesis of graphenes, carbon nanotubes, buckminsterfullerenes as well as polycyclic heteroaromatics.
Purification Methods
Purify triphenylene by zone refining or crystallisation from EtOH or CHCl3 and sublime. [Beilstein 5 IV 2556.]
Properties of Triphenylene
| Melting point: | 195-198 °C(lit.) |
| Boiling point: | 438 °C(lit.) |
| Density | 1.302 |
| refractive index | 1.5500 (estimate) |
| Flash point: | 438°C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in ethanol, benzene, acetic acid, oils and chloroform. |
| form | Crystalline Needles |
| color | White to beige |
| Water Solubility | 6.6ug/L(25.00 ºC) |
| λmax | 335nm(Hexane)(lit.) |
| Merck | 14,9740 |
| BRN | 1342908 |
| CAS DataBase Reference | 217-59-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Triphenylene(217-59-4) |
| IARC | 3 (Vol. Sup 7, 92) 2010 |
| EPA Substance Registry System | Triphenylene (217-59-4) |
Safety information for Triphenylene
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P501:Dispose of contents/container to..… |
Computed Descriptors for Triphenylene
| InChIKey | SLGBZMMZGDRARJ-UHFFFAOYSA-N |
Triphenylene manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 217-59-4 Triphenylene 98%View Details
217-59-4 Triphenylene 98%View Details
217-59-4 -
 Triphenylene 98% (HPLC) CAS 217-59-4View Details
Triphenylene 98% (HPLC) CAS 217-59-4View Details
217-59-4 -
 Triphenylene (purified by sublimation) CAS 217-59-4View Details
Triphenylene (purified by sublimation) CAS 217-59-4View Details
217-59-4 -
 Triphenylene CAS 217-59-4View Details
Triphenylene CAS 217-59-4View Details
217-59-4 -
 Triphenylene CAS 217-59-4View Details
Triphenylene CAS 217-59-4View Details
217-59-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1