Tripelennamine
- CAS NO.:91-81-6
- Empirical Formula: C16H21N3
- Molecular Weight: 255.36
- MDL number: MFCD00072167
- EINECS: 202-100-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-07 08:52:35
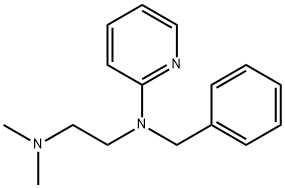
What is Tripelennamine?
Absorption
Well absorbed in the digestive tract.
Toxicity
Symptoms of overdose include clumsiness or unsteadiness, convulsions, drowsiness, dryness of mouth, nose, or throat, feeling faint, flushing or redness of face, hallucinations, muscle spasms (especially of neck and back), restlessness, shortness of breath or troubled breathing, shuffling walk, tic-like movements of head and face, trembling and shaking of hands and trouble in sleeping.
Chemical properties
White, bitter, crystalline powder. Solutions are acid to litmus.Soluble in water and alcohol; very slightly soluble in ether; practically insoluble in chloroform and benzene; 1% solution in water has a pH of 4.3.
Originator
Pyribenzamine,Ciba,US,1946
The Uses of Tripelennamine
This drug lessens the allergic response of the organism caused by histamine. Tripelennamine is used for allergic symptoms, rhinitis, conjunctivitis, and for allergic and anaphylactic reactions. Synonyms of this drug are pelanin and pyribenzamine.
The Uses of Tripelennamine
Medicine (antihistamine, sunburn treatment).
Indications
Used for the symptomatic relief of hypersensitivity reactions, coughs, and the common cold.
Background
A histamine H1 antagonist with low sedative action but frequent gastrointestinal irritation. It is used to treat asthma; HAY fever; urticaria; and rhinitis; and also in veterinary applications. Tripelennamine is administered by various routes, including topically.
Definition
ChEBI: Tripelennamine is an aromatic amine.
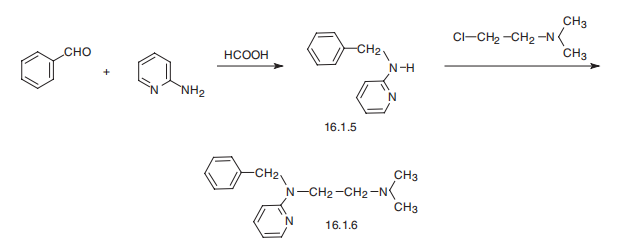
Manufacturing Process
46 g of α-benzylaminopyridine in 50 cc of dry toluene are heated to 80°C [the
α-benzylaminopyridine may be obtained either according to the method of
Tchitchibabine and Knunjanz, Berichte, 64, 2839 (1931), which consists in
warming α-aminopyridine with benzaldehyde in formic acid, or alternatively by
the action of benzyl chloride on sodio-α-aminopyridine]. To the toluene
solution there are added gradually 9.5 g of 85% sodamide. After evolution of
ammonia, the major part of the toluene is distilled off; into the pasty mass
which remains there are poured 120 cc of an ethereal solution of 27 g of
dimethylaminochloroethane.
The mixture is heated until the temperature reaches 140°C, the ether distilling
out, then finally heated under reduced pressure (150 mm Hg) for 1/2 hour.
The mass is taken up with dilute hydrochloric acid and ether, neutralized at pH
7, and α-benzylaminopyridine separates. After making alkaline, using excess of potash, it is extracted with benzene, dried and distilled. The product
thereby obtained, dimethylamino-ethyl-N-benzyl-N-α-arninopyridine, boils at
135° to 190°C/1.7 mm, according to US Patent 2,502,151.
brand name
PBZ (Novartis).
Therapeutic Function
Antihistaminic
Reactivity Profile
Tripelennamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
SYMPTOMS: Symptoms of exposure to Tripelennamine may include euphoria, aplastic anemia, excitement, hallucinations, ataxia, incoordination, athetosis, convulsions, postictal depression, dry mouth, fixed dilated pupils, flushing of the face, fever, central nervous system depression, drowsiness and coma.
Fire Hazard
Flash point data for Tripelennamine are not available. Tripelennamine is probably combustible.
Pharmacokinetics
Used to treat the effects of colds and allergies. Tripelennamine is an antihistamine. Histamine, acting on H1-receptors, produces pruritis, vasodilatation, hypotension, flushing, headache, tachycardia, and bronchoconstriction. Histamine also increases vascular permeability and potentiates pain. Tripelennamine is a histamine H1 antagonist. It competes with histamine for the normal H1-receptor sites on effector cells of the gastrointestinal tract, blood vessels and respiratory tract. It provides effective, temporary relief of sneezing, watery and itchy eyes, and runny nose due to hay fever and other upper respiratory allergies.
Safety Profile
Poison by ingestion and intraperitoneal routes. Human mutation data reported. Has been implicated in aplastic anemia. Used as an antdustasnine. Addicts have added it to paregoric to make "blue velvet," whtch can cause euphoria by injection. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
Tripelennamine, N-benzyl-N??,N??-dimethyl-N-2-pyridylethylenediamine (16.1.6), is synthesized by reacting 2-benzylaminopyrridine (16.1.5) with 2-dimethylaminoethylchloride in the presence of sodium amide. 2-Benzylaminopyrridine, in turn, can be easily synthesized by reduction of a Schiff base, synthesized by condensation of 2-aminopyrridine with benzaldehyde.
Metabolic pathway
When pyribenzamine is incubated with rat liver microsomes, it is metabolized via N-oxide formation and N-dealkylation which includes removal of the dimethylamino moiety, the thiophenylmethyl moiety of methaphenilene, and the benzyl moiety of pyribenzamine.
Metabolism
Hepatic
Properties of Tripelennamine
| Melting point: | 25°C |
| Boiling point: | bp0.1 138-142°; bp1.7 185-190°; bp20 193-205° |
| Density | 1.0683 (rough estimate) |
| refractive index | nD25 1.5759-1.5765 |
| pka | pKa 3.90±0.08(H2O
t undefined
I = 0.30
(NaCl)) (Uncertain);8.68±0.06(H2O
t undefined
I = 0.30
(NaCl)) (Uncertain) |
| Water Solubility | 587.3mg/L(30 ºC) |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | Tripelennamine (91-81-6) |
Safety information for Tripelennamine
Computed Descriptors for Tripelennamine
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![4-IODO-N-[2-[4-(METHOXYPHENYL)-1-PIPERAZINYL]ETHYL]-N-2-PYRIDINYL-BENZAMIDE HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/155204-23-2.gif)
![2-{[3-(6-METHYLPYRIDIN-2-YL)-4-OXO-3,4-DIHYDROQUINAZOLIN-2-YL]METHYL}-1H-ISOINDOLE-1,3(2H)-DIONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure5/GIF/CB1291685.gif)
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4