TRIOLEIN
Synonym(s):Triolein;Glyceryl trioleate;Glycerol trioleate;Glycerol triolein;Glyceryl trioleate solution
- CAS NO.:122-32-7
- Empirical Formula: C57H104O6
- Molecular Weight: 885.43
- MDL number: MFCD00137563
- EINECS: 204-534-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
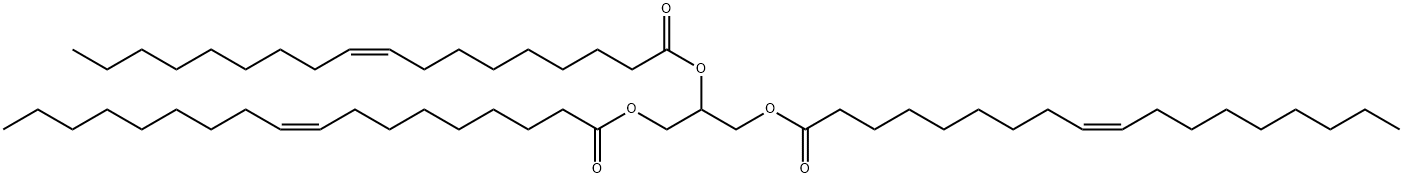
What is TRIOLEIN?
Description
1,2,3-Trioleoyl glycerol is a triacylglycerol that contains oleic acid (Item Nos. 90260 | 24659) at the sn-1, sn-2, and sn-3 positions. It inhibits oxidized LDL-induced decreases in cell viability and superoxide dismutase (SOD) and glutathione peroxidase (GPX) activities and increases in apoptosis in endothelial cells when used at a concentration of 10 μM. 1,2,3-Trioleoyl glycerol has been found in sunflower, corn, and extra virgin olive oils. It has been used as a standard for the quantification of triacylglycerols in extra virgin olive oil by high temperature gas chromatography-(ionic trap)mass spectrometry (HTGC-(IT)MS). 1,2,3-Trioleoyl glycerol is a major component of Lorenzo''s oil.
Description
Glyceryl trioleate, known widely as triolein, is an oily liquid that is a main constituent of some nondrying1 oils and fats. Triolein occurs in many natural fats and oils, including sunflower oil, palm oil, cacao butter, and, most notably, olive oil.
In 1941, Thomas P. Hilditch* and L. Maddison at the University of Liverpool (UK) crystallized olive oils from Italy and Palestine at temperatures down to –30 °C to resolve them into several components. They found that olive oil from Palestine contained ≈30% triolein, whereas the Italian oil contained only ≈5%.
Eight years later, M. L. Meara, also at Liverpool, resolved cacao butter into 11 fractions “by exhaustive crystallization”. For his efforts, only 1.1% of the fat turned out to be glyceryl trioleate. From 1940 to 1961, several chemists devised syntheses of triolein by esterifying glycerol and oleic acid.
Why do we celebrate glyceryl trioleate, and therefore olive oil, during the holiday season? Sacramental uses of olive oil are strongly connected to Christian and Jewish traditions, especially Hanukkah.
During the period of the Hanukkah story (168 BCE), only pure olive oil blessed by the high priest could be used to light the Temple menorah, which had to be lit continuously. After their victorious battle over the Syrian Greeks, the Maccabees could find only enough holy oil to last for one day. The Hanukkah miracle is that the oil lasted eight days, enough time for more oil to be prepared and sanctified.
Olive oil was the major component of anointing oils and lamp fuel that date to biblical times. Kings were anointed with oil as a mark of their official status; and one title for Jesus is the Anointed One. References are found throughout the Hebrew and Christian scriptures about the use of oil as part of fasting and healing rituals.
In Imani (faith), the seventh principle of Kwanzaa, designated persons are instructed to pour libations of water and oil into a bowl “for our ancestors; for the dying; for the glorious cloud of witnesses; and for life, health, and strength”.
And if you associate olive oil just with cooking, there are lots and lots of recipes available for cookies and cakes and muffins for your holiday baking!
1. “Nondrying” is somewhat of a misnomer; it means that the substance does not harden when it is exposed to air.
Chemical properties
colourless viscous liquid
Chemical properties
Triolein occurs as a clear, colorless to yellowish oily liquid, and is tasteless and odorless.
The Uses of TRIOLEIN
Glycerol trioleate can be used as an emulsifier, also as creams, lotions and lipsticks matrix. Used as textile finishing agent in textile industry, also used as additive in pigment grind. In pharm industry, used as ointment, emulsifiable paste, suppository, lotion, emulsifier in air agent, stabilizing agent, lubricating agent, antifoaming agents. In food industry, it has the function as emulsify, disperse, defoaming, blister, antistaling of starch and control fat agglutination, usually used as agent in candy, ice cream, cookie and bread. When used in beverage, could avoid ester to float, and avoid protein to sink, therefore to improve stability. When used in bread, cookie , could improve organization structure, increase volume, antistaling, prolong warranty validity.
The Uses of TRIOLEIN
Glyceryl trioleate has been used:
- as an experimental diet along with fat-free basal mix and corn oil and then to access the dietary fat absorption among mice
- as an interfering substance to test its effect on human serum in the approach to develop rapid enzyme immunoassay for the detection of retinol-binding protein
- as a standard in the determination of triglyceride concentration, colorimetrically using liver tissue sample from cows
Definition
ChEBI: A triglyceride formed by esterification of the three hydroxy groups of glycerol with oleic acid. Triolein is one of the two components of Lorenzo's oil.
Production Methods
Triolein is manufactured by the esterification of fractionated fatty acids, mainly oleic acid and glycerin.
General Description
Glyceryl trioleate is derived from glycerol. It is composed of three oleic acid units and is an unsaturated triglyceride.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Triolein is used as a solubilizer and solvent in injectable preparations.
It has been used in marketed preparations of sustained-release
injections of cytarabine and multivesicular liposomal injections of
morphine sulfate. It has also been used in enteric coatings for oral
preparations in combination with other enteric coating excipients to
protect against degradation by pancreatic lipase.
Triolen is used in personal care products as a skin-conditioning
and viscosity-controlling agent.
Safety
Triolein is used in injectable preparations, in enteric coatings for
oral preparations, and in personal care products. Chronic exposure
may cause nausea and vomiting, and higher exposures may cause
unconsciousness.
The Cosmetic Ingredient Review (CIR) Expert Panel found that
dermal application of triolein was not associated with significant
irritation, and no evidence of sensitization or photosensitization
was observed. Ocular exposures were found to be only mildly
irritating to eyes. Triolein has not been found to be genotoxic in a
number of in vitro and in vitro assay systems. Subcutaneous
injections of triolein in rats showed no tumors at the injection site.
The CIR Expert Panel also noted that metabolism data indicated
that glyceryl triesters (including triolein) followed the same
metabolic pathways as fats in food. They were split into
monoglycerides, free fatty acids, and glycerol, all of which were
absorbed into the intestinal mucosa and metabolized further.
Therefore, oral exposure to these compounds was not found to be
a concern.
A triolein-based amphotericin emulsion showed better safety
with a higher LD50 in rats as compared with the conventional
amphotericin deoxycholate.
Storage
Triolein is classified as a stable compound but is sensitive to air and light. It should be stored in tightly sealed containers in a dry area at 2–8℃. Thermal decomposition of triolein may lead to release of irritating gases and vapors such as carbon oxides. Exposure to air and moisture over prolonged periods should be avoided.
Incompatibilities
Triolein is incompatible with strong oxidizing agents and spontaneously flammable products. Being a triglyceride ester, triolein can be hydrolyzed by strong acids, and particularly by strong bases. It is possible for primary amines to form an adduct across the olefinic double bonds (analogous to a Michael addition).
Regulatory Status
Included in the FDA Inactive Ingredients Database (liposomal
suspension for epidural injections). Included in parenteral medicines
(suspension for intrathecal injection) licensed in the UK.
Triolein is included in the CIR category as safe for use in
cosmetics and personal care products. Its use as an indirect food
additive has been approved by the FDA.
Properties of TRIOLEIN
| Melting point: | -5,5°C |
| Boiling point: | 235-240 °C18 mm Hg(lit.) |
| Density | 0.91 g/mL(lit.) |
| refractive index | n |
| Flash point: | 330 °C |
| storage temp. | -20°C |
| solubility | chloroform: 0.1 g/mL, clear, colorless |
| form | Liquid |
| appearance | colorless to yellow oily liquid |
| color | Clear Pale Yellow |
| Merck | 14,9732 |
| BRN | 1718692 |
| Dielectric constant | 3.2(26℃) |
| Stability: | Stability Stable, but air and light sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 122-32-7(CAS DataBase Reference) |
| EPA Substance Registry System | 9-Octadecenoic acid (9Z)-, 1,1',1''-(1,2,3-propanetriyl) ester (122-32-7) |
Safety information for TRIOLEIN
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for TRIOLEIN
TRIOLEIN manufacturer
Spak Orgochem (India) Pvt. Ltd.
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
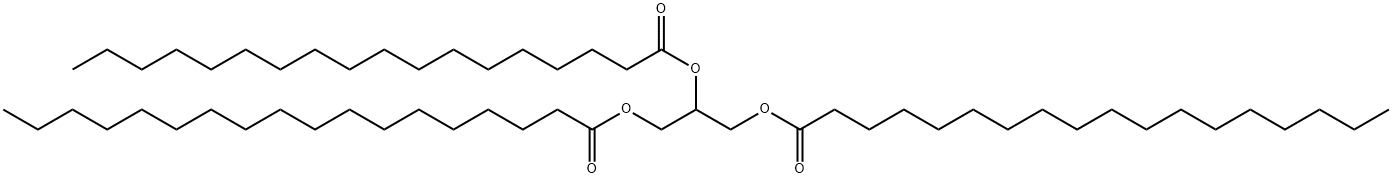
![TRIOLEIN, [CARBOXYL-14C]](https://img.chemicalbook.in/CAS/GIF/67318-71-2.gif)
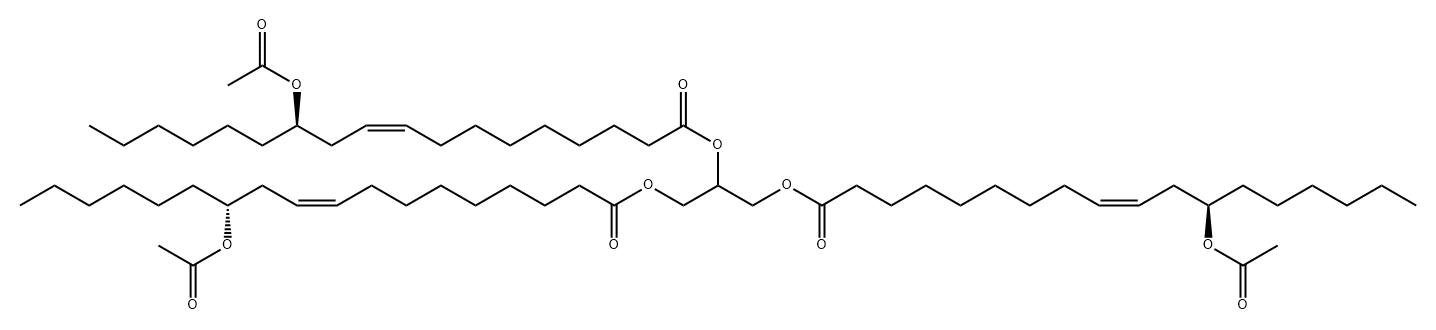
![TRIOLEIN, [9,10-3H(N)]](https://img.chemicalbook.in/CAS/GIF/70805-83-3.gif)


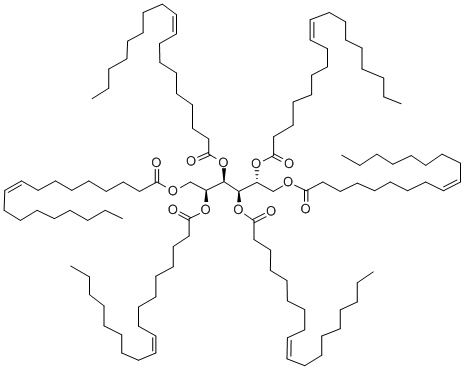
![TRIOLEIN, [GLYCEROL-1,2,3-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB7356159.gif)
You may like
-
 Triolein CAS 122-32-7View Details
Triolein CAS 122-32-7View Details
122-32-7 -
 Triolein CAS 122-32-7View Details
Triolein CAS 122-32-7View Details
122-32-7 -
 Triolein CAS 122-32-7View Details
Triolein CAS 122-32-7View Details
122-32-7 -
 Glyceryl trioleate, 60% CAS 122-32-7View Details
Glyceryl trioleate, 60% CAS 122-32-7View Details
122-32-7 -
 Triolein Reference Standard CAS 122-32-7View Details
Triolein Reference Standard CAS 122-32-7View Details
122-32-7 -
 Triolein CAS 122-32-7View Details
Triolein CAS 122-32-7View Details
122-32-7 -
 Triolein CAS 122-32-7View Details
Triolein CAS 122-32-7View Details
122-32-7 -
 Triolein CASView Details
Triolein CASView Details
