TriMethyl(broModifluoroMethyl)silane
- CAS NO.:115262-01-6
- Empirical Formula: C4H9BrF2Si
- Molecular Weight: 203.1
- MDL number: MFCD18641931
- EINECS: 806-938-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-08 16:52:38
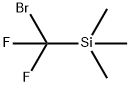
What is TriMethyl(broModifluoroMethyl)silane?
Chemical properties
Colorless transparent liquid, initial boiling point 108℃, relative density 1.31 g/cm3.
The Uses of TriMethyl(broModifluoroMethyl)silane
Trimethyl(bromodifluoromethyl)silane can be used as neuroactive steroids.
The Uses of TriMethyl(broModifluoroMethyl)silane
Novel difluorocarbene source for the efficient difluoromethylenation of alkenes/alkynes and difluoromethylation of O-, S-, and N-nucleophiles.
The Uses of TriMethyl(broModifluoroMethyl)silane
As reported by Hu and co-workers, (Bromodifluoromethyl)trimethylsilane is a highly useful reagent for the formation of difluoromethene-containing 3-membered rings and can also difluoromethylate heteroatoms with the assistance of alkaline bases, most effectively KOH.
What are the applications of Application
- TriMethyl(broModifluoroMethyl)silane commonly can be used as a source for generating difluorocarbene, it is a general reagent with broad synthetic utility.
- Can generate difluorocarbene under neutral/acidic/basic conditions in the presence/absence of water at low/high temperatures.
- Can be used to prepare gem-difluorocyclopropan(e)nes, O-, S-, N-, and P-difluoromethylated compounds
Reactions
(1) Difluoromethylenation of TMSCN.
Ref. J. Org. Chem. 2012, 77, 5850?5855.
(2) Difluoromethylenation of benzyl and alkylzinc halides.
Ref. Org. Lett. 2013, 15, 917 – 919.
(3) Fluorination aminocarbonylation of aldehydes
Ref. Angew. Chem. Int. Ed. 2022, e202115467
General Description
(Bromodifluoromethyl)trimethylsilane (TMSCF2Br) is commonly used as a source to generate dilfluorocarbene. TMSCF3 (Ruppert–Prakash reagent) and BBr3 undergoes fast halogen–exchange reaction to form MSCF2Br.
References
[1]Angewandte Chemie International Edition.DOI :10.1002/anie.202283811
[2]The Journal of Organic Chemistry.DOI :10.1021/acs.orglett.2c02899
Properties of TriMethyl(broModifluoroMethyl)silane
| Boiling point: | 108°C(lit.) |
| Density | 1.306 |
| refractive index | n/D1.407 |
| Flash point: | 46℃ |
| storage temp. | -20°C |
| form | liquid |
| color | clear |
| CAS DataBase Reference | 115262-01-6 |
Safety information for TriMethyl(broModifluoroMethyl)silane
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TriMethyl(broModifluoroMethyl)silane
| InChIKey | WDZVWBWAUSUTTO-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 115262-01-6 ( bromodifluoromethyl ) trimethylsilane 98%View Details
115262-01-6 ( bromodifluoromethyl ) trimethylsilane 98%View Details
115262-01-6 -
 Trimethyl(bromodifluoromethyl)silane 98% CAS 115262-01-6View Details
Trimethyl(bromodifluoromethyl)silane 98% CAS 115262-01-6View Details
115262-01-6 -
 (Bromodifluoromethyl)trimethylsilane CAS 115262-01-6View Details
(Bromodifluoromethyl)trimethylsilane CAS 115262-01-6View Details
115262-01-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
