Trimethobenzamide
- CAS NO.:138-56-7
- Empirical Formula: C21H28N2O5
- Molecular Weight: 388.46
- MDL number: MFCD00599601
- EINECS: 205-332-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-17 18:22:24
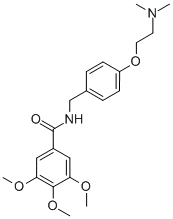
What is Trimethobenzamide?
Absorption
The relative bioavailability of the capsule formulation compared to the solution is 100%.
Toxicity
Oral LD50 in mice is 1600 mg/kg.
Originator
Tigan,Beecham,US,1973
The Uses of Trimethobenzamide
Antiemetic.
Indications
For the treatment of postoperative nausea and vomiting and for nausea associated with gastroenteritis.
Background
Trimethobenzamide is a novel antiemetic which prevents nausea and vomiting in humans. Its actions are unclear but most likely involves the chemoreceptor trigger zone (CTZ). In dogs pretreated with trimethobenzamide HCl, the emetic response to apomorphine is inhibited, while little or no protection is afforded against emesis induced by intragastric copper sulfate.
Definition
ChEBI: The amide obtained by formal condensation of 3,4,5-trihydroxybenzoic acid with 4-[2-(N,N-dimethylamino)ethoxy]benzylamine. It is used to prevent nausea and vomitting in humans.
Manufacturing Process
To 122 grams (1 mol) of p-hydroxybenzaldehyde in 1 liter of chlorobenzene
were added 66 grams (1.04 mols) of sodium methoxide (85%) and 108 grams
(1 mol) of 2-dimethylaminoethyl chloride. The mixture was stirred and
refluxed for 15 hours, then cooled and the precipitated sodium chloride
filtered off. The filtrate was concentrated at steam temperature under water
vacuum and the residual oil was fractionated in high vacuum, to give 4-(2-
dimethylaminoethoxy)benzaldehyde, BP2.2145°C.
Two teaspoons of Raney nickel catalyst were added to a solution of 65.6
grams (0.34 mol) of 4-(2-dimethylaminoethoxy)benzaldehyde in 300 ml of
10% ammoniacal ethanol. The mixture was hydrogenated at 80°C and a
pressure of 1,000 psi. The catalyst was filtered off, the volatiles were distilled
off and the residual oil was fractionated in high vacuum, to obtain 4-(2-
dimethylaminoethoxy)benzylamine, BP0.3120° to 123°C.
To 9.7 grams (0.05 mol) of 4-(2-dimethylaminoethoxy)benzylamine, dissolved
in 100 ml of acetonitrile, was added all at once 12 grams (0.051 mol) of
3,4,5-trimethoxybenzoyl chloride, dissolved in 75 ml of acetonitrile. The
mixture was stirred and refluxed for 8 hours, and then cooled. The crystalline
solid, which had formed, was filtered off, washed with acetonitrile and
recrystallized from acetonitrile, to give 4-(2-dimethylaminoethoxy)-N-(3,4,5-
trimethoxybenzoyl)benzylamine hydrochloride, MP 185° to 186°C.
brand name
Tigan (King).
Therapeutic Function
Antinauseant
Pharmacokinetics
Trimethobenzamide is a novel antiemetic which prevents nausea and vomiting in humans. Its actions are unclear but most likely involves the chemoreceptor trigger zone (CTZ). In dogs pretreated with trimethobenzamide HCl, the emetic response to apomorphine is inhibited, while little or no protection is afforded against emesis induced by intragastric copper sulfate.
Metabolism
Hepatic.
Properties of Trimethobenzamide
| storage temp. | 2-8°C |
| pka | pKa 8.27±0.03(H2O) (Uncertain) |
| NIST Chemistry Reference | Benzamide, n-[[4-[2-(dimethylamino)ethoxy]phenyl]methyl]-3,4,5-trimethoxy-(138-56-7) |
Safety information for Trimethobenzamide
Computed Descriptors for Trimethobenzamide
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 138-56-7 Trimethobenzamide 98%View Details
138-56-7 Trimethobenzamide 98%View Details
138-56-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
