Trigonelline
- CAS NO.:535-83-1
- Empirical Formula: C7H7NO2
- Molecular Weight: 137.14
- MDL number: MFCD00054262
- EINECS: 208-620-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
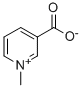
What is Trigonelline?
Description
Trigonelline is a bitter alkaloid in coffee which serves to produce important aroma compounds. In terms of concentration trigonelline is higher for arabica than robusta and ranges from about 0.6-1.3% and 0.3-0.9%, respectively.
Trigonelline, the N-methylpyridinium-3-carboxylate, is, after caffeine, the second most important alkaloid of coffee, with about 1% of the green bean. During leaf development, it is synthesized in the leaves and in the fruits' pericarp and accumulated in the seeds. The direct precursors are nicotinic acid and nicotine amide, deriving from the pyridine nucleotide cycle.
Trigonelline is a plant hormone that has diverse regulatory functions with respect to plant cell cycle regulation, nodulation, and oxidative stress, and in helping survival and growth of the plant. It is found in significant quantity in many plants like coffee beans and fenugreek seed. Because it was first isolated from the fenugreek seeds (Trigonella foenum-graecum), the name assigned to it has been “trigonelline.” The chemical formula for trigonelline is C7H7NO2. It is a methylation product of niacin (vitamin B3), and thus is also known as “methylated niacin.” At higher temperatures, trigonelline breaks down to niacin. In addition to trigonelline, fenugreek seeds contain other alkaloids such as gentianine and carpaine.
Chemical properties
White solid
The Uses of Trigonelline
antihyperglycemic.
Trigonelline is an inhibitor which, like abscicic acid, accumulates in plant cells when the plant becomes dormant or is exposed to stress. This natural inhibitor is chemically related to glycine betaine. It has been found in dormant winter buds of Populus trichocarpa * deltoides trees in the field as well as in salt-stressed micropropagules thereof. Some trigonelline also occurred in non-stressed micropropagules, probably as the result of bioconversion of niacin taken up from the nutrient medium (Bray et al. 1988). Trigonelline preferentially promotes arrest in G2 of the cell cycle and appears to have a growth controlling function during seed germination (Evans and Tramontano 1981).
The Uses of Trigonelline
Trigonelline is an alkaloid present in coffee and fenugreek presenting phytoestrogenic activity.
Definition
ChEBI: An iminium betaine that is the conjugate base of N-methylnicotinic acid, arising from deprotonation of the carboxy group.
Biosynthesis
Trigonelline is synthesized by the methylation of nicotinic acid. This reaction is catalyzed by Sadenosyl-L-methionine (SAM) dependent nicotinate N-methyltransferase (EC 2.1.1.7), which is found in crude extracts of the pea. This enzyme has now been purified from heterotrophic cultured cells and leaves of Glycine max. The Km values for nicotinate and SAM were 78 μM and 55 μM, respectively in the enzyme derived from cultured cells, and 12.5 μM and 31 μM in leaves. The optimum pH of the cultured cell enzyme is alkaline (8.0), but that of the leaf enzyme is acidic (6.5). The gene encoding trigonelline synthase has not yet been cloned from any organism.
Purification Methods
Crystallise trigonelline (as monohydrate) from aqueous EtOH, then dry it at 100o. It also crystallises from H2O as the monohydrate with m 230-233o(dec). It has been crystallised from EtOH with m 214-215o(dec). The picrate crystallises from EtOH with m 204-206o. [Green & Tong J Am Chem Soc 78 4896 1956, Kosower & Patton J Org Chem 26 1319 1961, Beilstein 22 III/IV 462, 22/2 V 143.]
References
Jahns., Ber., 18,2518 (1885)
Thoms., ibid, 31,271,404 (1891)
Schultz, Frankfurt., ibid, 27,709 (1894)
Gorter., Annalen, 372,237 (1910)
Schultz, Trier., Zeit. physiol. Chem., 76,258 (1912)
Holtz, Kutscher, Theilmann., Zeit. Bioi., 8, 57 (1924)
Rimington., Onderstepoort J., 5, 81 (1935)
Pharmacology :
Ackermann., Zeit. Bioi., 59, 17 (1912)
Volmer, Furst., Bull. Acad. Med., 122,241 (1939)
Properties of Trigonelline
| Melting point: | 260°C (dec.) |
| Boiling point: | 251.96°C (rough estimate) |
| Density | 1.2528 (rough estimate) |
| refractive index | 1.5030 (estimate) |
| storage temp. | Refrigerator |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | Off-White to Light Beige |
Safety information for Trigonelline
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trigonelline
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Trigonelline 98.00% CAS 535-83-1View Details
Trigonelline 98.00% CAS 535-83-1View Details
535-83-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1