Rosmarinic acid
Synonym(s):(R)-O-(3,4-Dihydroxycinnamoyl)-3-(3,4- dihydroxyphenyl)lactic acid;3,4-Dihydroxycinnamic acid (R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl ester;Rosmarinic acid
- CAS NO.:20283-92-5
- Empirical Formula: C18H16O8
- Molecular Weight: 360.31
- MDL number: MFCD00017740
- EINECS: 606-487-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
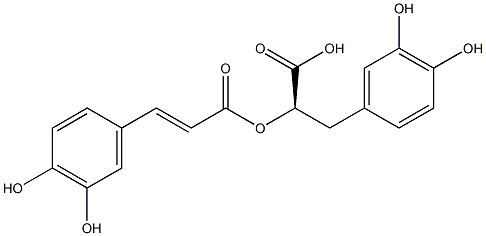
What is Rosmarinic acid?
Description
Rosmarinic acid (20283-92-5) displays radical scavenging1, anti-inflammatory2 and amyloid-β aggregation inhibition activities.3 Rosmarinic acid has also been shown to act as an agonist at GPR35.4
Description
Carnosic acid (top two images) is a diterpene with an abietane skeleton; rosmarinic acid (bottom two) is a caffeic acid ester.?Both are found in the rosemary plant (Rosmarinus officinalis), each also occurs in other plants.
But carnosic and rosmarinic acids have something else in common:?They are meat preservatives. Rosemary extract was originally added to meat as a flavoring agent, but people noticed that it kept meat from spoiling. It was later discovered that the acids inhibit the free-radical reaction that oxidizes fats and oils and causes spoilage. Neither acid is responsible for the rosemary flavor.
Chemical properties
White to off-white powder
The Uses of Rosmarinic acid
Rosmarinic Acid is a phenolic compound with antioxidant and anti-inflammatory activity. Rosmarinic Acid has been shown to inhibit againts peroxidative damage to biomembranes. Rosmarinic Acid also supp resses endotoxin-induced activation of complement and concomitant prostacyclin biosynthesis.
The Uses of Rosmarinic acid
An activator of GPR35.
The Uses of Rosmarinic acid
Rosmarinic acid is a naturally-occurring phenolic compound with antioxidant and anti-inflammatory properties. This compound inhibits lipid peroxidation of rat liver microsomes by 90% at a concentration of 25 μg/ml. Rosmarinic acid suppresses endotoxin-induced activation of complement and concomitant formation of prostacyclin. Formation of 5-HETE and LTB4 from human PMNL is inhibited by rosmarinic acid at concentrations of 10-5 to 10-3 M.
What are the applications of Application
Rosmarinic Acid is an activator of GPR35
Definition
ChEBI: (R)-rosmarinic acid is a stereoisomer of rosmarinic acid having (R)-configuration. It has a role as a plant metabolite and a geroprotector. It is a conjugate acid of a (R)-rosmarinate. It is an enantiomer of a (S)-rosmarinic acid.
General Description
Rosmarinic acid is a bioactive plant phenolic compound with antioxidant and anti-inflammatory effects.
Biological Activity
Anti-inflammatory, cytostatic and antiviral.
Biochem/physiol Actions
Rosmarinic acid has shown to contain antioxidant, anti-inflammatory and antimicrobial activities. Possesses promising physiological actions related to cognitive performance, Alzheimer′s disease prevention, kideney disease treatment, cardioprotection and cancer chemoprevention.
Mode of action
Rosmarinic acid (RA), a water-soluble polyphenolic compound with antioxidant and anti-inflammatory properties. Inhibits several complement-dependent inflammatory processes via inhibition of the C5 convertase. Anticarcinogenic. Inhibitor of lipid peroxidation, TCR-induced T cell activation and proliferation.?
References
1) Miliauskas et al. (2005), Identification of radical scavenging compounds in Rhaponticum carthamoides by means of LC-DAD-SPE-NMR; J. Nat. Prod., 68 168 2) Sahu et al. (1999), Inhibition of complement by covalent attachment of rosmarinic acid to activated C3b; Biochem. Pharmacol, 57 1439 3) Ono et al. (2012), Phenolic compounds prevented amyloid β-protein oligomerization and synaptic dysfunction by site-specific binding; J. Biol. Chem., 287 14631 4) Deng et al. (2012), Multiple tyrosine metabolites are GPR35 agonists; Sci. Rep., 2 373
Properties of Rosmarinic acid
| Melting point: | 171-175 °C (lit.) |
| Boiling point: | 694.7±55.0 °C(Predicted) |
| alpha | +102~+110°(D/20℃)(c=0.2,C2H5OH) |
| Density | 1.33 |
| storage temp. | 2-8°C |
| solubility | Soluble in ethanol, DMSO or dimethyl formamide to approximately 25 mg/mL. |
| form | powder |
| pka | 2.78±0.10(Predicted) |
| color | white to faintly beige |
| BRN | 2227587 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 1 month. |
| InChI | InChI=1/C18H16O8/c19-12-4-1-10(7-14(12)21)3-6-17(23)26-16(18(24)25)9-11-2-5-13(20)15(22)8-11/h1-8,16,19-22H,9H2,(H,24,25)/b6-3+/t16-/s3 |
| CAS DataBase Reference | 20283-92-5(CAS DataBase Reference) |
Safety information for Rosmarinic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Rosmarinic acid
| InChIKey | DOUMFZQKYFQNTF-WUTVXBCWSA-N |
| SMILES | C(C1C=CC(O)=C(O)C=1)[C@H](C(=O)O)OC(=O)/C=C/C1C=CC(O)=C(O)C=1 |&1:9,r| |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 20283-92-5 Rosmarinic acid 99%View Details
20283-92-5 Rosmarinic acid 99%View Details
20283-92-5 -
 20283-92-5 99%View Details
20283-92-5 99%View Details
20283-92-5 -
 Rosmarinic acid 98%View Details
Rosmarinic acid 98%View Details
20283-92-5 -
 Rosmarinic acid 20283-92-5 98%View Details
Rosmarinic acid 20283-92-5 98%View Details
20283-92-5 -
 Rosmarinic acid, 96% CAS 20283-92-5View Details
Rosmarinic acid, 96% CAS 20283-92-5View Details
20283-92-5 -
 Rosmarinic acid 98% CAS 20283-92-5View Details
Rosmarinic acid 98% CAS 20283-92-5View Details
20283-92-5 -
 Rosmarinic acid CAS 20283-92-5View Details
Rosmarinic acid CAS 20283-92-5View Details
20283-92-5 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
