Trifluralin
Synonym(s):2,6-Dinitro-N,N-dipropyl-4-trifluoromethylaniline
- CAS NO.:1582-09-8
- Empirical Formula: C13H16F3N3O4
- Molecular Weight: 335.28
- MDL number: MFCD00055241
- EINECS: 216-428-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
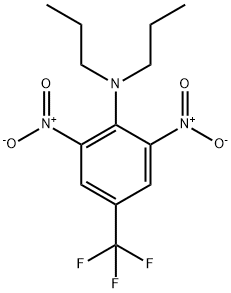
What is Trifluralin?
Description
Trifluralin is a dinitroaniline herbicide that is approved for preemergence use to control broadleaf weeds in a variety of crops and plants. First approved nearly 50 years ago, trifluralin is a common commercially available herbicide which is used extensively in the United States and other countries. In the early 2000s, trifluralin was banned in Europe following reports of persistence in soil and groundwater leading to concerns of increased risk for toxicity. In general, trifluralin is viewed as a safe herbicide when used according to the instructions. Minimal to no toxicity has been reported in humans following either oral, dermal, or inhalation exposure. There is some evidence that once trifluralin enters the soil and groundwater, it undergoes a complex and extensive series of metabolic steps and can exist as multiple intermediaries depending on the extent of the degradation. This has led to findings that trifluralin may have elevated toxicity in certain aquatic wildlife as particular species of fish and tadpoles have displayed biomarkers of trifluralin toxicity following exposure. Larger vertebrates such as canines have also demonstrated toxicological profiles suggesting an elevated toxicity compared to humans.
Chemical properties
Yellowish-orange solid. Insoluble in water; soluble in xylene, acetone, and ethanol.
Chemical properties
Trifluralin is an orange crystalline solid.
The Uses of Trifluralin
Trifluralin is a herbicide, first approved in 1963, for control of annual grasses and broadleaf weeds on a variety of crops. Trifluralin is registered for nonfood uses including residential use. Trifluralin comes in a variety of formulations and is applied as a soil-incorporated treatment.
The Uses of Trifluralin
Pre-emergence herbicide used for grass control in crops.
The Uses of Trifluralin
Preemergence herbicide for controlling many grasses and broad-leaved weeds.
Definition
ChEBI: A substituted aniline that is N,N-dipropylaniline substituted by a nitro groups at positions 2 and 6 and a trifluoromethyl group at position 4. It is an agrochemical used as a pre-emergence herbicide.
General Description
Yellow-orange crystalline solid. Denser than water and not soluble in water. Hence sinks in water. Melting point 48.5-49°C. Used as a selective pre emergence herbicide.
Reactivity Profile
Trifluralin is a trifluoromethyl dinitroaniline derivative. Dinitroaniline has a record of industrial explosions. Nothing has been reported implicating Trifluralin in any such accidents. This may be because of the trifluoromethyl substitution may mitigate the instability of the molecule. However, care should be taken in the extremes of heat, shock, and friction sources that may trigger an explosive release of energy.
Health Hazard
Dust may irritate eyes. No toxic symptoms have been observed during the manufacture and use of Trifluralin.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating hydrogen fluoride gas may be formed in fires.
Agricultural Uses
Herbicide: Trifluralin is a selective, pre-emergence herbicide used to control many annual grasses and broadleaf weeds in a large variety of tree fruit, nut, vegetable, and grain crops, including soybeans, sunflowers, cotton, and alfalfa. It is also used on rights-of-way, on set-aside land (i.e., arable land temporarily taken out of use). A general use pesticide (GUP). Not approved for use in EU countries . Registered for use in the U.S.
Trade name
AGREFLAN®; AGRIFLAN® 24; ASHLADE TRIMARAN®; AUTUMN KITE®; BROADSTRIKE®; BUCKLE®; CALLIFORT®; CAMPBELL'S TRIFLURON®; CHANDOR®; COMMENCE®; CRISALIN®; DEVRINOL T®; DIGERMIN®; ELANCOLAN®; FLINT®; FLORA®; FLURENE SE®; FLUTRIX®; FREEDOM®; GORDON’S WEEDER®; HERBIFLURIN®; IPERSAN®; JANUS®; L-36352®; LILLY 36,352®; LINNET®; MARKSMAN®; MARKSMAN 2, TRIGARD®; M. T. F®; NITRAN®; OLITREF®; ONSLAUGHT®; PREMERLIN 600 CE®; SINFLOWAN®; SOLO®; SU SEGURO CARPIDOR®; TEAM®; TREFANOCIDE®; TREFICON®; TREFLAN®; TREFLANOCIDE®; TRIFARMON®; TRIFLURALINA® 600; TRIFLUREX®; TRIFUREX®; TRIGARD®; TRIKEPIN®; TRILIN®; TRILIN® 10G; TRIM®; TRIMARAN®; TRIPART TRIFLURALIN 48 EC; TRISTAR®; TRUST®; TURFLAN®; URANUS® (trifluralin + linuron)
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Human mutation data reported. When heated to decomposition it emits very toxic fumes of Fand NOx. See also FLUORIDES.
Potential Exposure
A potential danger to those involved in the manufacture, formulation and application of this selective, preemergence herbicide.
Environmental Fate
Biological. Laanio et al. (1973) incubated
14CF3-tri?uralin with Paecilomyces, Fusar-
ium oxysporum, or Aspergillus fumigatus and reported that <1% was converted to
14CO2.
From the first-order biotic and abiotic rate constants of tri?uralin in estuarine water and
sediment/water systems, the estimated biodegradation half-lives were 2.5–9.7 and 2.4–7.1
days, respectively (Walker et al., 1988).
Soil. Anaerobic degradation in a Crowley silt loam yielded α,α,α-tri?uoro-N4,N4-
dipropyl-5-nitrotoluene-3,4-diamine and α,α,α-tri?uoro-N4,N4-dipropyltoluene-3,4,5-tri-
amine (Parr and Smith, 1973). Probst and Tepe (1969) reported that tri?ur
Golab et al. (1979) studied the degradation of tri?uralin in soil over a 3-year period.
They found that the herbicide undergoes N-dealkylation, reduction of nitro substituents,
followed by the formation cyclized products. Of the 28 transformation products
Zayed et al. (1983) studied the degradation of tri?uralin by the microbes Aspergillus
carneus, Fusarium oxysporum and Trichoderma viride. Following an inoculation and
incubation period of 10 days in the dark at 25°C, the following metabolites were identified:
α,α,α-trifluoro-2,6-dinitro-N-propyl-p-toluidine, α,α,α-trifluoro-2,6-dinitro-p-toluidine,
2-amino-6-nitro-α,α,α-tri?uoro-p-toluidine and 2,6-dinitro-4-tri?uoromethyl phenol. The
reported half-life in soil is 132 days (Jury et al., 1987).
The half-lives for tri?uralin in soil incubated in the laboratory under aerobic and
anaerobic conditions ranged from 33 to 375 days (Probst et al., 1967; Parr and Smith,
1973; Kearney et al., 1976; Zimdahl and Gwynn, 1977) to 4 to 70 days, respectively
(
Solubility in organics
Freely soluble in Stoddard solvent (Windholz et al., 1983), chloroform, methanol (Probst et al., 1967), acetone (400 g/L), xylene (580 g/L) (Worthing and Hance, 1991), ether, and ethanol (quoted, Bailey and White, 1965)
Shipping
UN2588 Pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Toxicity evaluation
Technical trifluralin has low acute toxicity, whereas solvents often used for the emulsification of trifluralin have been shown to be irritating to the eyes and skin. An active component of trifluralin toxicity is the volatile nitrosamine, N-nitroso-din- propylamine, which may be the active compound in trifluralin toxicity.
Incompatibilities
Trifluralin is a trifluoromethyl dinitroaniline derivative. Dinitroaniline has a record of industrial explosions. Nothing has been reported implicating trifluralin in any such accidents. This may be because of the trifluoromethyl substitution may mitigate the instability of the molecule. However, care should be taken in the extremes of heat, shock, and friction sources that may trigger an explosive release of energy. Fluorocarbons can react violently with barium, potassium, sodium.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Trifluralin does contain fluorine, and therefore incineration presents the increased hazard of HF in the off-gases. Prior to incineration, fluorine-containing compounds should be mixed with slaked lime plus vermiculite, sodium carbonate or sand-soda ash mixture (90-10).
Properties of Trifluralin
| Melting point: | 48.5°C |
| Boiling point: | 139°C |
| Density | 1.294 |
| vapor pressure | 1.97 at 30 °C (effusion method, DePablo, 1976) |
| Flash point: | 100 °C |
| storage temp. | APPROX 4°C
|
| solubility | DMSO : ≥ 100 mg/mL (298.26 mM);Water : < 0.1 mg/mL (insoluble) |
| pka | -1.45±0.50(Predicted) |
| form | Crystalline Solid |
| color | Bright orange |
| Water Solubility | <0.01 g/100 mL at 22.5 ºC |
| Merck | 13,9757 |
| BRN | 1893555 |
| Henry's Law Constant | 10.19 and 14.89 in distilled water and 33.3‰ NaCl at 20 °C, respectively (wetted-wall column,
Rice et al., 1997a) |
| CAS DataBase Reference | 1582-09-8(CAS DataBase Reference) |
| IARC | 3 (Vol. 53) 1991 |
| NIST Chemistry Reference | Trifluralin(1582-09-8) |
| EPA Substance Registry System | Trifluralin (1582-09-8) |
Safety information for Trifluralin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Trifluralin
Trifluralin manufacturer
Gujarat Chemicals GUJCHEM
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 1582-09-8 Trifluralin 99%View Details
1582-09-8 Trifluralin 99%View Details
1582-09-8 -
 Trifluralin 95% CAS 1582-09-8View Details
Trifluralin 95% CAS 1582-09-8View Details
1582-09-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1