Trifluoroacetic anhydride
Synonym(s):Bis(trifluoroacetic) anhydride, TFAA;TFAA;Trifluoroacetic anhydride
- CAS NO.:407-25-0
- Empirical Formula: C4F6O3
- Molecular Weight: 210.03
- MDL number: MFCD00000416
- EINECS: 206-982-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
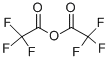
What is Trifluoroacetic anhydride?
Chemical properties
Clear, colorless liquid
The Uses of Trifluoroacetic anhydride
Used as analytical reagents, solvents, catalysts, dehydration condensing agent, protection agent of trifluoroacetylation of carboxyl and amino; used as Intermediate of fluorine fine chemicals, pharmaceutical, agrochemical.
The Uses of Trifluoroacetic anhydride
Trifluoroacetic anhydride is used for the introduction of trifluoroacetyl group in organic synthesis. It is involved in the preparation of N- and O-trifluoroacetyl derivatives of a wide range of biologically active compounds for gas chromatography analysis. It plays an important role as desiccant for trifluoroacetic acid. It is also used in the oxidation of aldehydes to acids, esters, amides as well as in the protection of alcohols and amines. In addition, it is used as analytical reagents, solvents and dehydration condensing agent. It serves as an intermediate of fluorine fine chemicals, pharmaceuticals and agrochemicals.
The Uses of Trifluoroacetic anhydride

To a solution of the SM (1.0 g, 4 mmol) in dry DCM (20 mL) at 0 C was added TEA (3.1 g, 31.0 mmol), followed by TFAA (4.1 g, 20.0 mmol). The resulting mixture was allowed to warm to RT and stir for 16 h, after which time it was partitioned between DCM (50 mL) and H2O (50 mL). The org layer was washed with sat aq NaHCO3 (100 mL), dried (Na2SO4), and concentrated in vacuo to provide the product as a thick liquid. [800 mg, 87%]
What are the applications of Application
Trifluoroacetic anhydride is for the preparation of N- and O-trifluoroacetyl derivatives
Safety Profile
A severe skin and eye irritant.Explosive reaction with dimethyl sulfoxide; nitric acid +1,3,5-triazine (at 36°C); nitric acid + 1,3,5-triacetylhexahydro-1,3,5-triazine (at 30°C). Incompatiblewith lithium tetrahydroaluminate. When heated todecomposit
Purification Methods
Purification by distilling over KMnO4, as for the acid above, is EXTREMELY DANGEROUS due to the possiblility of EXPLOSION. It is best purified by distilling from P2O5 slowly, and collecting the fraction boiling at 39.5o. Store it in a dry atmosphere. Highly TOXIC vapour and attacks skin, work in an efficient fume hood. [Beilstein 2 IV 469.]
Properties of Trifluoroacetic anhydride
| Melting point: | -65 °C (lit.) |
| Boiling point: | 39.5-40 °C (lit.) |
| Density | 1.511 g/mL at 20 °C (lit.) |
| vapor pressure | 6.28 psi ( 20 °C) |
| refractive index | n |
| Flash point: | -26 °C |
| storage temp. | 2-8°C |
| solubility | Miscible with benzene, dichloromethane, diethyl ether, dimethylformamide, terahydrofuran and acetonitrile. |
| form | Liquid |
| appearance | Colorless liquid |
| pka | 0.43[at 20 ℃] |
| Specific Gravity | 1.487 |
| color | Clear |
| Water Solubility | Hydrolysis |
| Sensitive | Moisture Sensitive |
| BRN | 746197 |
| Stability: | Stable, but moisture sensitive. Reacts with water to liberate hydrogen (flammable!). Incompatible with water, strong oxidizing agents. |
| CAS DataBase Reference | 407-25-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, trifluoro-, anhydride(407-25-0) |
| EPA Substance Registry System | Acetic acid, trifluoro-, anhydride (407-25-0) |
Safety information for Trifluoroacetic anhydride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H332:Acute toxicity,inhalation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Trifluoroacetic anhydride
Trifluoroacetic anhydride manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
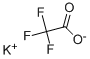
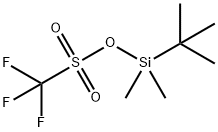
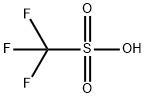
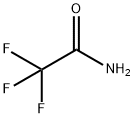
You may like
-
 Trifluoroacetic anhydride 98%View Details
Trifluoroacetic anhydride 98%View Details -
 Trifluoroacetic Anhydride CAS 407-25-0View Details
Trifluoroacetic Anhydride CAS 407-25-0View Details
407-25-0 -
 Trifluoroacetic anhydride CAS 407-25-0View Details
Trifluoroacetic anhydride CAS 407-25-0View Details
407-25-0 -
 Trifluoroacetic anhydride, (GC) 99% CAS 407-25-0View Details
Trifluoroacetic anhydride, (GC) 99% CAS 407-25-0View Details
407-25-0 -
 Trifluoroacetic anhydride CAS 407-25-0View Details
Trifluoroacetic anhydride CAS 407-25-0View Details
407-25-0 -
 Trifluoroacetic anhydride CAS 407-25-0View Details
Trifluoroacetic anhydride CAS 407-25-0View Details
407-25-0 -
 Trifluoroacetic Acid Anhydride CASView Details
Trifluoroacetic Acid Anhydride CASView Details -
 TRIFLUOROACETIC ANHYDRIDE CAS Number: 407-25-0View Details
TRIFLUOROACETIC ANHYDRIDE CAS Number: 407-25-0View Details
407-25-0
