Tricaine methanesulfonate
Synonym(s):MS-222;Tricaine;Tricaine methanesulfonate;TS 222
- CAS NO.:886-86-2
- Empirical Formula: C9H11NO2.CH4O3S
- Molecular Weight: 261.3
- MDL number: MFCD00013176
- EINECS: 212-956-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-09-04 16:42:00
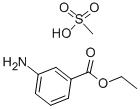
What is Tricaine methanesulfonate?
Chemical properties
WHITE TO OFF-WHITE FINE POWDER
The Uses of Tricaine methanesulfonate
The anesthetic of choice for amphibians is tricaine methanesulfonate (MS - 222, tricaine, FINQUEL, Argent Chemical Laboratories, Redmond, WA) (Wright 2001f ). It is a white crystalline powder that may be mixed with water to anesthetize fi sh and amphibians. Amphibians are immersed in a bath of tricaine methanesulfonate until anesthesia is achieved and then maintained in fresh water for the particular procedure to be performed. For longer procedures, the patient may be immersed in a 50% dilution of the original induction solution or intubated and maintained on isofl urane.
The Uses of Tricaine methanesulfonate
A benzocaine isomer derivative.
The Uses of Tricaine methanesulfonate
Ethyl 3-Aminobenzoate Methanesulfonate is used as anesthetic for broodfish such as rainbow trout and red pacu. Ethyl 3-Aminobenzoate Methanesulfonate also acts as the sodium channel blocker which reverses the degenerative effects of fipronil insecticide.
What are the applications of Application
Ethyl 3-aminobenzoate methanesulfonate is a benzocaine isomer derivative
Definition
ChEBI: A methanesulfonate salt obtained by reaction of tricaine with one molar equivalent of methanesulfonic acid. Used as an anaesthetic for fish.
General Description
Ethyl 3-aminobenzoate methanesulfonate is mainly used as an anesthetic for fish. It is also used for the euthanasia of all fish species especially zebrafish, which is commonly used in research.
Biological Activity
ethyl 3-aminobenzoate can block the generation of action potentials via voltage-dependent na+-channels.sodium channels play a critical role in physiology: they can rapidly transmit depolarizing impulses throughout cells and cell networks, therefore enabling co-ordination of higher processes. sodium channels are also of special importance for the history of physiology.
in vitro
the major mode of action for ethyl 3-aminobenzoate was found to be nervous system suppression whereby the entrance of sodium into the nerve was inhibited, therefore limiting nerve membrane excitability. in addition, the nerve inhibition was facilitated by the lipid solubility of ethyl 3-aminobenzoate, which allowed it to move easily into the cell membrane to bind with sodium channels [1].
in vivo
previous study evaluated the potency and dynamics of ethyl 3-aminobenzoate-induced effects on neuronal firing of sensory and motor nerves alongside a defined motor behavior in xenopus laevis tadpoles. electrophysiological recordings of extraocular motor discharge and nerve activity were measured before, during and after administration of drugs. results showed that ethyl 3-aminobenzoate and benzocaine, but not pancuronium could cause a dose-dependent, reversible blockade of extraocular motor and sensory nerve activity. such results indicated that ethyl 3-aminobenzoate was able to block the activity of both sensory and motor nerves compatible with the mechanistic action of effective anesthetics, suggesting that ethyl 3-aminobenzoate was effective as single-drug anesthetic for surgical interventions in anamniotes [2].
References
[1] k. m. carter, c. m. woodley and r. s. brown. a review of tricaine methanesulfonate for anesthesia of fish. rev. fish biol. fisheries 21, 51-59 (2011).
[2] c. ramlochansingh, f. branoner, b. p. chagnaud, et al. efficacy of tricaine methanesulfonate (ms-222) as an anesthetic agent for blocking sensory-motor responses in xenopus laevis tadpoles. plos one 9(7), e101606 (2014).
Properties of Tricaine methanesulfonate
| Melting point: | 146-149 °C(lit.) |
| Density | 1.3799 (rough estimate) |
| refractive index | 1.5060 (estimate) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | Fine Powder |
| color | White to off-white |
| Merck | 14,9621 |
| BRN | 3775022 |
| CAS DataBase Reference | 886-86-2(CAS DataBase Reference) |
| EPA Substance Registry System | Benzoic acid, 3-amino-, ethyl ester, methanesulfonate (886-86-2) |
Safety information for Tricaine methanesulfonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tricaine methanesulfonate
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 Ethyl 3-aminobenzoate CAS 886-86-2View Details
Ethyl 3-aminobenzoate CAS 886-86-2View Details
886-86-2 -
 Tricaine methanesulfonate 98% CAS 886-86-2View Details
Tricaine methanesulfonate 98% CAS 886-86-2View Details
886-86-2 -
 Tricaine CAS 886-86-2View Details
Tricaine CAS 886-86-2View Details
886-86-2 -
 Ethyl 3-aminobenzoate methanesulfonate salt CAS 886-86-2View Details
Ethyl 3-aminobenzoate methanesulfonate salt CAS 886-86-2View Details
886-86-2 -
 Ethyl 3-aminobenzoate methanesulfonate CAS 886-86-2View Details
Ethyl 3-aminobenzoate methanesulfonate CAS 886-86-2View Details
886-86-2 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4