ETHYL ANTHRANILATE
Synonym(s):Benzocaine impurity D (PhEur);Ethyl anthranilate
- CAS NO.:87-25-2
- Empirical Formula: C9H11NO2
- Molecular Weight: 165.19
- MDL number: MFCD00007711
- EINECS: 201-735-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
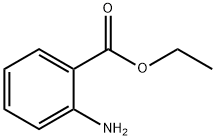
What is ETHYL ANTHRANILATE?
Chemical properties
colourless liquid
Chemical properties
Ethyl anthranilate has a faint, orange-flower odor and similar taste.
Occurrence
Reported found in grapes, orange juice, orange peel oil, starfruit and Vitis labrusca L.
The Uses of ETHYL ANTHRANILATE
Perfumery and flavors, similar to methyl anthranilate.
The Uses of ETHYL ANTHRANILATE
Ethyl 2-aminobenzoate is used as a reactant in the amination of aryl chlorides, bromides, and triflates.
Preparation
By esterification of anthranilic acid with ethanol in the presence of acid catalysts; by reacting sodium hypochlorite with an alkaline solution of phthalimide
Definition
ChEBI: Ethyl 2-aminobenzoate is a benzoate ester.
General Description
Colorless liquid with a fruity odor. Insoluble in water.
Air & Water Reactions
ETHYL ANTHRANILATE should be protected from air and light. Insoluble in water.
Reactivity Profile
ETHYL ANTHRANILATE may hydrolyze under acidic and basic conditions.
Fire Hazard
Flash point data for ETHYL ANTHRANILATE are not available. ETHYL ANTHRANILATE is probably combustible.
Pharmacology
Ethyl anthranilate behaved as a local anaesthetic, as measured by depression of
muscle twitch, but was 50% less effective than the m- and p-aminobenzoate isomers. All three compounds
potentiated the initial phase of caffeine-induced contracture of frog sartorius muscle, but
the o-isomer had no effect on the peak tension of the contracture (Friedman, Bianchi & Weiss.
1974). It was proposed that the o-amino group has both steric and polar effects, its bulk preventing
the inhibitory action of the carbonyl group on the caffeine-induced contracture of sartorius muscle,
while the lone electron pair of the nitrogen atom induces a contracture on its own accord and
potentiates a caffeine-induced contracture (Friedman, 1975).
Ethyl anthranilate (0-1 mmol/litre) decreased the frequency of the electric-organ discharges of
the electric fish, Gnathonemus moori, but did not affect the individual pulse amplitudes, indicating
that the compound acted on the pacemaker cells of the mesencephalic command nucleus (Walsh
& Schopp, 1966).
Safety Profile
Moderately toxic by ingestion. A skin irritant. Combustible liquid. When heated to decomposition it emits toxic fumes of NOx.
Properties of ETHYL ANTHRANILATE
| Melting point: | 13-15 °C (lit.) |
| Boiling point: | 129-130 °C/9 mmHg (lit.) |
| Density | 1.117 g/mL at 25 °C (lit.) |
| vapor density | 5.7 (vs air) |
| refractive index | n |
| FEMA | 2421 | ETHYL ANTHRANILATE |
| Flash point: | >230 °F |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Insoluble in water but soluble in organic solvents |
| form | Clear liquid |
| pka | 2.20±0.10(Predicted) |
| Specific Gravity | 1.1170 |
| color | Light yellow |
| Odor | at 100.00 %. sweet floral grape orangeblossom wintergreen |
| JECFA Number | 1535 |
| BRN | 878874 |
| Stability: | Stable. Combustible. Incompatible with acids, bases, oxidizing agents. |
| CAS DataBase Reference | 87-25-2(CAS DataBase Reference) |
| EPA Substance Registry System | Ethyl anthranilate (87-25-2) |
Safety information for ETHYL ANTHRANILATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for ETHYL ANTHRANILATE
ETHYL ANTHRANILATE manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1-GLYCERYL N-[7-CHLORO-4-QUINOLYL]ANTHRANILATE HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/3820-67-5.gif)


You may like
-
 ETHYL-2-AMINO BENZOATE 98%View Details
ETHYL-2-AMINO BENZOATE 98%View Details
87-25-2 -
 Ethyl anthranilate 87-25-2 98%View Details
Ethyl anthranilate 87-25-2 98%View Details
87-25-2 -
 Ethyl-2-Amino Benzoate 97% CAS 87-25-2View Details
Ethyl-2-Amino Benzoate 97% CAS 87-25-2View Details
87-25-2 -
 Ethyl anthranilate CAS 87-25-2View Details
Ethyl anthranilate CAS 87-25-2View Details
87-25-2 -
 Ethyl 2-Aminobenzoate CAS 87-25-2View Details
Ethyl 2-Aminobenzoate CAS 87-25-2View Details
87-25-2 -
 ETHYL ANTHRANILATE For Synthesis CAS 87-25-2View Details
ETHYL ANTHRANILATE For Synthesis CAS 87-25-2View Details
87-25-2 -
 Ethyl 2-aminobenzoate CAS 87-25-2View Details
Ethyl 2-aminobenzoate CAS 87-25-2View Details
87-25-2 -
 Ethyl 2-aminobenzoate CAS 87-25-2View Details
Ethyl 2-aminobenzoate CAS 87-25-2View Details
87-25-2