Triazolam
Synonym(s):8-Chloro-6-[2-chlorophenyl]-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
- CAS NO.:28911-01-5
- Empirical Formula: C17H12Cl2N4
- Molecular Weight: 343.21
- MDL number: MFCD00079623
- EINECS: 249-307-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
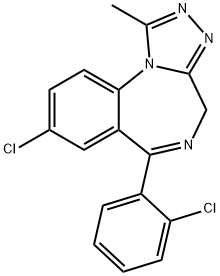
What is Triazolam?
Absorption
Bioavailability is 44% (oral) and 53% (sublingual).
Toxicity
Symptoms of overdose include drowsiness, slurred speech, motor inco-ordination, coma, and respiratory depression.
Chemical properties
Yellow Solid
Originator
Halcion,Upjohn,Switz.,1978
The Uses of Triazolam
Triazolam
The Uses of Triazolam
Sedative, hypnotic. Controlled substance (depressant).
Background
Withdrawn in the United Kingdom due to risk of psychiatric adverse drug reactions. This drug continues to be available in the U.S. Internationally, triazolam is a Schedule IV drug under the Convention on Psychotropic Substances.
Indications
For the short-term treatment of insomnia.
Definition
ChEBI: Triazolam is a triazolobenzodiazepine. It has a role as a sedative.
Manufacturing Process
A mixture of 1.0g (0.0031 mol) of 7-chloro-1,3-dihydro-5-(o-chlorophenyl)- 2H-1,4-benzodiazepine-2-thione, 0.8 g (0.0108 mol) of acetic acid hydrazide and 40 ml of 1-butanol was heated at reflux temperature under nitrogen for 24 hours. During the first 5 hours the nitrogen was slowly bubbled through the solution. After cooling and removing the solvent in vacuo, the product was well mixed with water and collected on a filter, giving 0.9 g of orange solid, melting point 210°C to 212°C. This was heated under nitrogen in an oil bath at 250°C and then cooled, The solid was crystallized from ethyl acetate, giving 0.5 g of tan solid of melting point 215°C to 216°C (decomposition). This was dissolved in 25 ml of 2-propanol, filtered, concentrated to10 ml and cooled, yielding 0.46 g (43%) of tan, crystalline 8-chloro-1-methyl-6-(o_x0002_chlorophenyl)-4H-s-triazolo[4,3-a][1,4]-benzodiazepine of melting point 223°C to 225°C.
brand name
Novoderm;Nuctane;Songarn.
Therapeutic Function
Hypnotic
World Health Organization (WHO)
Triazolam, a benzodiazepine derivative with sedative and hypnotic activity, was introduced in 1978 for themanagement of insomnia. It is controlled under Schedule IV of the 1971 Convention of Psychotropic Substances. Concern regarding the psychotropic effects of triazolam was first raised in the Netherlands in 1979 when this compound was suspended for sale and subsequently withdrawn by the Committee for the Evaluation of Medicines on the basis of reports of a reversible complex of symptoms including paranoia, depersonalization, nightmares, suicidal tendency and hyperaesthesia in patients receiving the drug. The basis for this decision was later successfully contested by the manufacturer and the drug was reregistered in early 1990 with a revised product information. However, concern was regenerated elsewhere that higher doses are associated with an unacceptable incidence of unwanted effects and the manufacturer has eventually withdrawn 0.5 mg tablets on a worldwide basis. In 1991 the issue of the safety of triazolam was again reopened by reports of retrograde amnesia and depression among patients taking the decreased recommended dosages. The product information has been revised by the United States FDA to include more rigorous cautions regarding dosage. In the Member States of the European Communities the products have been suspended pending further review by the EC Committee on Proprietary Medicinal Products.
General Description
Triazolam, 8-chloro-6-(o-chlorophenyl)-1-methyl-4H-s-triazolo[4,3-a][1,4] benzodiazepine(Halcion), has all of the characteristic benzodiazepine pharmacologicalactions. It is an ultra–short-acting hypnoticbecause it is rapidly α-hydroxylated to the 1-methyl alcohol,which is then rapidly conjugated and excreted.Consequently, it has gained popularity as sleep inducers, especiallyin elderly patients, because it causes less daytimesedation. It is metabolically inactivated primarily by hepaticand intestinal CYP3A4; therefore, coadministration withgrapefruit juice increases its peak plasma concentration by30%, leading to increased drowsiness.
Pharmacokinetics
A short-acting benzodiazepine used as a hypnotic agent in the treatment of insomnia. Some countries temporarily withdrew triazolam from the market because of concerns about adverse reactions, mostly psychological, associated with higher dose ranges. Its use at lower doses with appropriate care and labeling has been reaffirmed by the FDA and most other countries. Triazolam has a shorter half-life than chlordiazepoxide, flurazepam, and prazepam and does not generate active metabolites.
Metabolism
Hepatic. Small amounts of unmetabolized triazolam appear in the urine.
Properties of Triazolam
| Melting point: | 209-212°C |
| Boiling point: | 499.51°C (rough estimate) |
| Density | 1.2835 (rough estimate) |
| refractive index | 1.6300 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | DMF: 30 mg/ml; DMF:PBS(pH 7.2)(1:1): 0.5 mg/ml; DMSO: 20 mg/ml; Ethanol: 10 mg/ml |
| form | A crystalline solid |
| pka | pKa 1.52(H2O) (Uncertain);6.5(H2O) (Uncertain) |
| Water Solubility | 30mg/L(ambient temperature) |
| CAS DataBase Reference | 28911-01-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Triazolam(28911-01-5) |
| EPA Substance Registry System | Triazolam (28911-01-5) |
Safety information for Triazolam
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Triazolam
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1