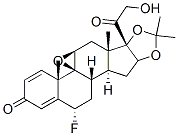
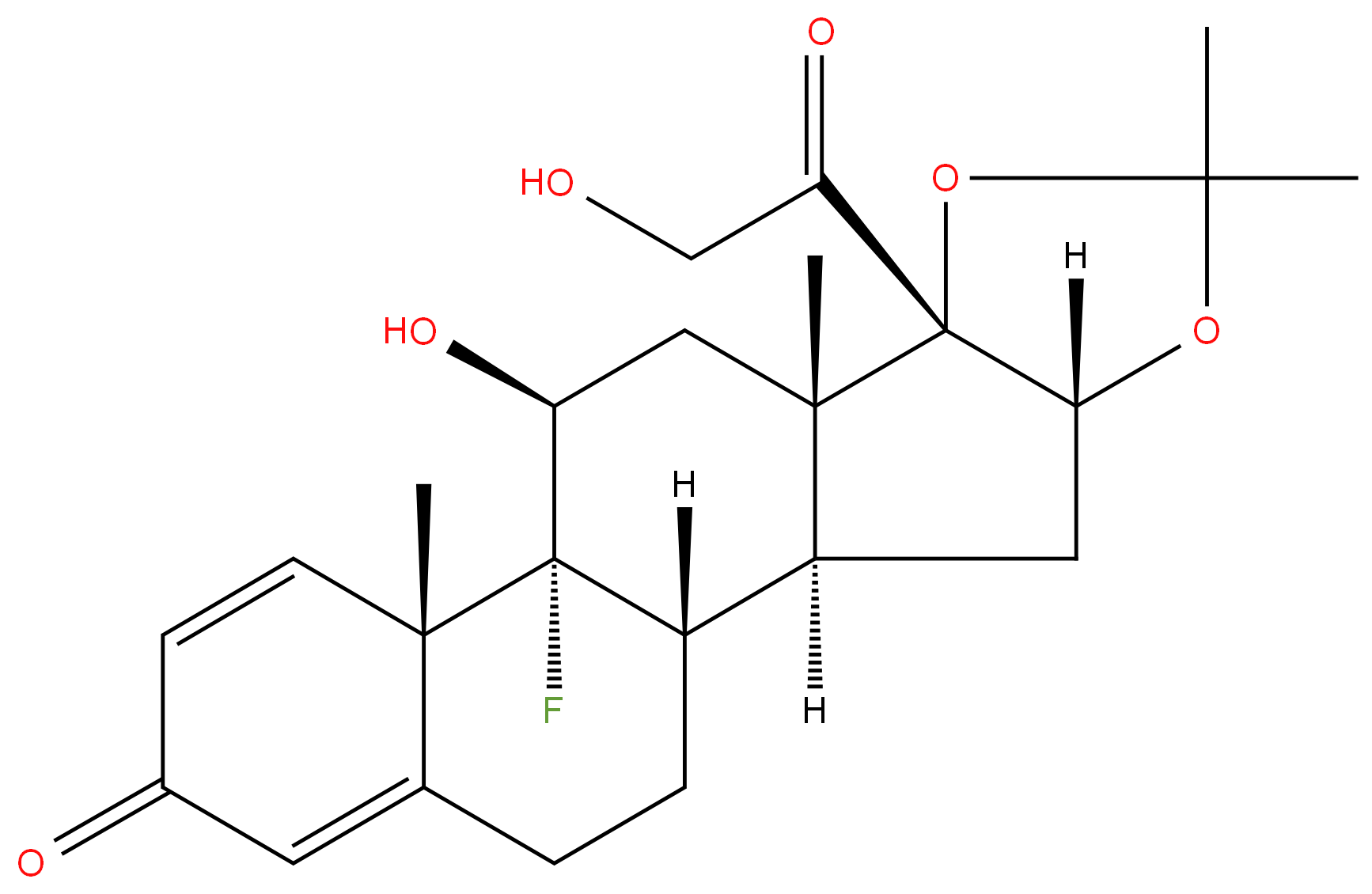
Triamcinolone acetonide
Synonym(s):9α-Fluoro-11β,16α,17α,21-tetrahydroxy-1,4-pregnadiene-3,20-dione 16,17-acetonide;9α-Fluoro-16α-hydroxyprednisolone 16α,17α-acetonide;Triamcinolone acetonide
- CAS NO.:76-25-5
- Empirical Formula: C24H31FO6
- Molecular Weight: 434.5
- MDL number: MFCD00056834
- EINECS: 200-948-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:12:03
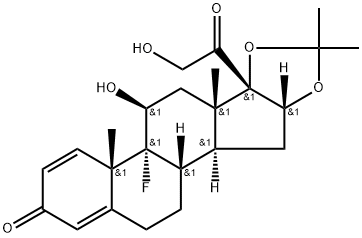
What is Triamcinolone acetonide?
Description
Triamcinolone acetonide is a synthetic corticosteroid. It decreases cytokine levels, the firing rate of sensory neurons, and mechanical hypersensitivity in a rat spinal nerve ligation model when used at a dose of 1.5 mg/kg prior to and following surgery for three days. Triamcinolone acetonide also decreases outflow facility in a mouse model of steroid-induced glaucoma when 20 μl of a 40 mg/ml suspension is administered subconjunctivally. Formulations containing triamcinolone acetonide are used in the treatment of diabetic macular edema.
Chemical properties
White Solid
Originator
Kenalog,Squibb,US,1958
The Uses of Triamcinolone acetonide
Triamcinolone acetonide is a topical and systemic corticosteroid belonging to the group-B (triamcinolone acetonide) type of steroids.
Definition
ChEBI: A synthetic glucocorticoid that is the 16,17-acetonide of triamcinolone. Used to treat various skin infections.
Indications
Triamcinolone acetonide (Aristocort, Kenalog) is a synthetic fluorinated corticosteroid.
Manufacturing Process
A solution of 250 mg of 9α-fluoro-11β,16α,17α,21-tetrahydroxy-1,4- pregnadiene-3,20-dione in 70 ml of hot acetone and 7 drops of concentrated hydrochloric acid is boiled for 3 minutes. After standing at room temperature for 17 hours, the reaction mixture is poured into dilute sodium bicarbonate and extracted with ethyl acetate. The extract is washed with saturated saline solution, dried and evaporated to a colorless glass. The residue is crystallized from acetone-petroleum ether to afford 166 mg of the acetonide, MP 270° to 274°C, decomposition, (with previous softening and browning). Three recrystallizations from acetone-petroleum ether give 113 mg of 9α-fluoro- 11β,21-dihydroxy-16α,17α-isopropylidenedioxy-1,4-pregnadiene-3,20-dione, MP 274° to 279°C, decomposition, (with previous softening and browning).
brand name
Triamcinolone acetonide was sold under the brand name Kenalog among others.
Therapeutic Function
Glucocorticoid
Mechanism of action
Triamcinolone acetonide is a corticosteroid. It is specifically a glucocorticoid, or an agonist of the glucocorticoid receptor, that is about five times as potent as cortisol. It has very little mineralocorticoid effects.The affinities of triamcinolone acetonide for the androgen and estrogen receptors are both <0.1% (relative to testosterone and estradiol).
General Description
The three main metabolitesof triamcinolone acetonide (Azmacort, Nasacort) are 6β-hydroxytriamcinolone acetonide, 21-carboxytriamcinoloneacetonide, and 6β-hydroxy-21-carboxytriamcinolone acetonide.All are much less active than the parent compound.The 6β-hydroxyl group and the 21-carboxy group are bothstructural features that greatly reduce GC action. The increasedwater solubility of these metabolites also facilitatesmore rapid excretion.
General Description
Triamcinolone acetonide is approximately 8 times morepotent than prednisone in animal inflammation models.Topically applied triamcinolone acetonide is a potent antiinflammatoryagent, about 10 times more sothan triamcinolone. The plasma half-life is approximately 90minutes, although the plasma half-life and biological halflivesfor GCs do not correlate well. The hexacetonide isslowly converted to the acetonide in vivo and is given onlyby intra-articular injection. Only triamcinolone and the diacetateare given orally. The acetonide and diacetate may begiven by intra-articular or intrasynovial injection. In addition,the acetonide may be given by intrabursal or, sometimes,IM or subcutaneous injection. A single IM dose of thediacetate or acetonide may last up to 3 or 4 weeks. Plasmalevels with IM doses of the acetonide are significantly higherthan with triamcinolone itself. The acetonide is also used totreat asthma and allergic rhinitis.
Clinical Use
Triamcinolone acetonide used topically to treat various skin conditions, to relieve the discomfort of mouth sores, and by injection into joints to treat various joint conditions.Triamcinolone acetonide frequently is used by inhalation for the treatment of lung diseases (e.g., asthma).
Adverse reactions
Significant: Adrenal suppression, immunosuppression (e.g. secondary infections, superinfections); kaposi sarcoma, myopathy, psychiatric disturbances including insomnia and depression (parenteral); local topical, nasal or ocular effects (e.g. sensitization, visual disturbances such as increased intraocular pressure, endophthalmitis).
Eye disorders: Cataract (ocular).
Gastrointestinal disorders: Dyspepsia, tooth disorder (nasal).
Injury, poisoning and procedural complications: Injection site reactions (e.g. blurring of vision for ocular, discomfort, pain, erythema, swelling, necrosis, abscess).
Investigations: Elevated blood pressure, weight gain (parenteral).
Metabolism and nutrition disorders: Increased appetite, fluid retention, glucose tolerance alteration (parenteral).
Musculoskeletal and connective tissue disorders: Arthralgia (parenteral).
Nervous system disorders: Headache.
Respiratory, thoracic and mediastinal disorders: Epistaxis (nasal).
Potentially Fatal: Rarely, anaphylaxis.
Side Effects
The side effects of using Triamcinolone acetonide include:Skin dryness, flaking, crusting, burning, or blistering; Skin irritation; Skin soreness, itching, swelling, scaling, or severe redness; Scaling or redness near mouth; Skin thinning or bruising, especially in skin folds (like between the finger) or on the face (when directed to use it there); New or worsening pimples or acne; Skin burning and itching with tiny red blisters; Skin softening; Itching, pain, or burning sensation in hairy areas, or pus at the root of the hair; Increased hair growth on the legs, arms, back, or forehead; Lightening of skin tone; Red or purple lines on the arms, face, legs, groin, or trunk.
Safety Profile
Poison by subcutaneous and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and toxic fumes of F-.
Veterinary Drugs and Treatments
The systemic veterinary labeled product (Vetalog? Injection) is labeled
as “indicated for the treatment of inflammation and related
disorders in dogs, cats, and horses. It is also indicated for use in
dogs and cats for the management and treatment of acute arthritis,
allergic and dermatologic disorders.”
Glucocorticoids have been used in an attempt to treat practically
every malady that afflicts man or animal, but there are three broad
uses and dosage ranges for use of these agents. 1) Replacement of
glucocorticoid activity in patients with adrenal insufficiency, 2)
as an antiinflammatory agent, and 3) as an immunosuppressive.
Among some of the uses for glucocorticoids include treatment of: endocrine conditions (e.g., adrenal insufficiency), rheumatic diseases
(e.g., rheumatoid arthritis), collagen diseases (e.g., systemic
lupus), allergic states, respiratory diseases (e.g., asthma), dermatologic
diseases (e.g., pemphigus, allergic dermatoses), hematologic
disorders (e.g., thrombocytopenias, autoimmune hemolytic anemias),
neoplasias, nervous system disorders (increased CSF pressure),
GI diseases (e.g., ulcerative colitis exacerbations), and renal
diseases (e.g., nephrotic syndrome). Some glucocorticoids are used
topically in the eye and skin for various conditions or are injected
intra-articularly or intra-lesionally. The above listing is certainly
not complete.
Metabolism
Triamcinolone acetonide frequently is used by inhalation for the treatment of lung diseases (e.g., asthma). After inhalation, triamcinolone acetonide can become systemically available when the inhaled formulation is swallowed and absorbed unchanged from the GI tract, causing undesirable systemic effects. Triamcinolone acetonide that is swallowed is metabolized to 6β-hydroxytriamcinolone acetonide, 21-carboxytriamcinolone acetonide, and 21-carboxy-6β-hydroxytriamcinolone acetonide, all of which are more hydrophilic than their parent drug. Only approximately 1% of the dose was recovered from the urine as triamcinolone acetonide. Triamcinolone is not a major metabolite of triamcinolone acetonide in humans, suggesting that acetonide is resistant to hydrolytic cleavage. Triamcinolone acetonide is approximately eight times more potent than prednisolone.
Properties of Triamcinolone acetonide
| Melting point: | 274-278°C (dec.) |
| alpha | D23 +109° (c = 0.75 in chloroform) |
| Boiling point: | 576.9±50.0 °C(Predicted) |
| Density | 1.1517 (estimate) |
| refractive index | 1.5980 (estimate) |
| storage temp. | Refrigerator |
| solubility | Practically insoluble in water, sparingly soluble in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| pka | 12.87±0.10(Predicted) |
| color | White to Off-White |
| Water Solubility | Soluble in DMSO or ethanol. Slightly soluble in water. |
| Merck | 9596 |
| Stability: | Combustible. Incompatible with strong oxidizing agents. |
| NIST Chemistry Reference | Triamcinolone acetonide(76-25-5) |
| EPA Substance Registry System | Triamcinolone acetonide (76-25-5) |
Safety information for Triamcinolone acetonide
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Triamcinolone acetonide
| InChIKey | YNDXUCZADRHECN-JNQJZLCISA-N |
Triamcinolone acetonide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
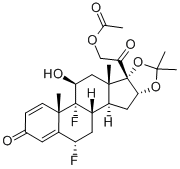
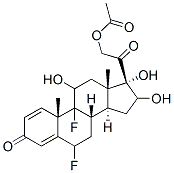
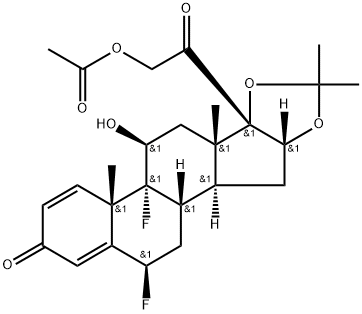
You may like
-
 Triamcinolone Acetonide 98%View Details
Triamcinolone Acetonide 98%View Details -
 Triamcinolone acetonide 99%View Details
Triamcinolone acetonide 99%View Details -
 Triamcinolone acetonide 99%View Details
Triamcinolone acetonide 99%View Details
76-25-5 -
 Triamcinolone Acetonide CAS 76-25-5View Details
Triamcinolone Acetonide CAS 76-25-5View Details
76-25-5 -
 Triamcinolone acetonide 95.00% CAS 76-25-5View Details
Triamcinolone acetonide 95.00% CAS 76-25-5View Details
76-25-5 -
 Triamcinolone acetonide 99% (HPLC) CAS 76-25-5View Details
Triamcinolone acetonide 99% (HPLC) CAS 76-25-5View Details
76-25-5 -
 Triamcinolone Acetonide CAS 76-25-5View Details
Triamcinolone Acetonide CAS 76-25-5View Details
76-25-5 -
 Triamcinolone acetonide CAS 76-25-5View Details
Triamcinolone acetonide CAS 76-25-5View Details
76-25-5
