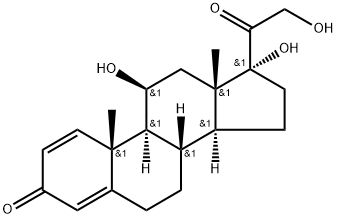
Triamcinolone
Synonym(s):9α-Fluoro-11β,16α,17,21-tetrahydroxy-1,4-pregnadiene-3,20-dione;9α-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione;Fluoxyprednisolone
- CAS NO.:124-94-7
- Empirical Formula: C21H27FO6
- Molecular Weight: 394.43
- MDL number: MFCD00010477
- EINECS: 204-718-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
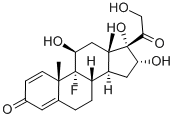
What is Triamcinolone ?
Absorption
A 16mg oral dose of triamcinolone reaches a Cmax of 5.23±0.84ng/mL with a Tmax of 2.24±0.78h and an AUC of 36.0±6.2ng*h/mL.
A 2mg intravenous dose of triamcinolone acetonide has an AUC of 57.7ng*h/mL. The bioavailability of 800μg of inhaled triamcinolone acetonide is 25%, with 10.4% coming from pulmonary absorption and the rest being accounted for by deposition on the oral mucosa and other underlying factors. An inhaled dose of triamcinolone acetonide reaches a Cmax of 0.92ng/mL with a Tmax of 1.74h and an AUC of 5.12ng*h/mL. The fraction of an inhaled dose that is actually absorbed via the pulmonary route reaches a Cmax of 0.55ng/mL with a Tmax of 0.66h and an AUC of 2.15ng*h/mL.
A 16mg oral dose of triamcinolone diacetate reaches a Cmax of 5.33±1.55ng/mL with a Tmax of 1.86±0.47h and an AUC of 32.7±9.9ng*h/mL.
Toxicity
The subcutaneous LD50 of triamcinolone acetonide in rats is 13,100μg/kg and in mice is 132mg/kg. The oral LD50 in rats is 1451mg/kg and in mice is 2168mg/kg.[LD50] The intraperitoneal LD50 in mice is 105mg/kg.[LD50]
Patients experiencing an overdose may develop Cushing's syndrome. This overdose may be treated with supportive therapy and mifepristone for its antiglucocorticoid activity.
Description
A natural extension of corticoid research involved examination of compounds containing both a 9α-fluoro group and a double bond between positions 1 and 2. Triamcinolone (9-fluoro-11β, 16α, 17, 21-tetrahydroxypregna- 1,4-diene-3,20-dione), introduced in 1958, combines the structural features of a ?1 -corticoid and a 9α-fluoro corticoid. As mentioned previously, the 9α-fluoro group increases the anti-inflammatory potency, but it also markedly increases the mineralocorticoid potency. This is undesirable if the drug is to be used internally for the treatment of rheumatoid arthritis. By inserting a 16α-hydroxy group into the molecule, one can decrease the mineralocorticoid activity.
Chemical properties
White, crystalline powder. Insoluble in water; slightly soluble in usual organic solvents; soluble in dimethylformamide.
Originator
Kenacort,Squibb,US,1958
The Uses of Triamcinolone
Triamcinolone is a glucocorticoid. Triamcinolone is used as an antiasthmatic (inhalant); antiallergic (nasal).
The Uses of Triamcinolone
A glucocorticoid, antiasthmatic (inhalant); antiallergic (nasal).
Background
Triamcinolone is a corticosteroid used to treat various inflammatory conditions in the body from allergic rhinitis to acute exacerbations of multiple sclerosis. Triamcinolone can be used as a one time adjunct treatment of osteoarthritic knee pain, or first line as a topical treatment of corticosteroid responsive dermatoses. Triamcinolone is more commonly seen in the forms triamcinolone hexacetonide, triamcinolone acetonide, and triamcinolone diacetate.
Triamcinolone was granted FDA approval on 3 December 1957. In October 2021, a suspension of triamcinolone acetonide was approved for suprachoroidal injection - the first suprachoroidal injection to receive FDA approval - for the treatment of patients with macular edema associated with uveitis.
Indications
Triamcinolone hexacetonide injections are indicated for intralesional administration in alopecia areata, discoid lupus erythematosus, keloids, and necrobiosis lipoidica diabeticorum. This formulation can also be used for localized hypertrophic infiltrated inflammatory lesions of granuloma annulare, lichen planus, lichen simplex chronicus, and psoriatic plaques.
Triamcinolone acetonide spray and cream are indicated for the treatment of inflammatory and pruritic manifestations of corticosteroid responsive dermatoses. A triamcinolone acetonide 10mg/mL or 40mg/mL injection is indicated intra-articularly for acute gouty arthritis, acute and subacute bursitis, acute nonspecific tenosynovitis, epicondylitis, rheumatoid arthritis, and synovitis of osteoarthritis. The same 10mg/mL injection is indicated by the intralesional route for the treatment of alopecia areata, discoid lupus erythematosus, keloids, necrobiosis lipoidica diabeticorum, and tumors of an aponeurosis or tendon. This formulation can also be used for localized hypertrophic infiltrated inflammatory lesions of granuloma annulare, lichen planus, lichen simplex chronicus, and psoriatic plaques. The 40mg/mL injection is indicated intramuscularly for controlling severe allergic conditions such as asthma, atopic dermatitis, contact dermatitis, drug hypersensitivity, perennial or seasonal allergic rhinitis, serum sickness, and transfusion reactions; treatment of bullous dermatitis herpetiformis, exfoliative erythroderma, mycosis fungoides, pemphigus, Stevens-Johnson syndrome, congenital adrenal hyperplasia, hypercalcemia in
cancer, nonsuppurative thyroiditis, autoimmune hemolytic anemia, Diamond-Blackfan anemia, pure red cell aplasia, secondary thrombocytopenia, trichinosis, tuberculous meningitis, acute exacerbations of multiple sclerosis or cerebral edema, sympathetic ophthalmia, temporal arteritis, uveitis, ocular inflammation, berylliosis, idiopathic eosinophilic pneumonias, symptomatic sarcoidosis, dermatomyositis, polymyositis, and systemic lupus erythematosus; adjunct treatment of adrenocortical insufficiency, regional enteritis, ulcerative colitis, fulminating or disseminated pulmonary tuberculosis, acute gouty arthritis, acute rheumatic carditis, ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis; palliative management of leukemia and lymphoma; induction of diuresis or remission of proteinuria in idiopathic nephrotic syndrome or lupus erythematosus. A triamcinolone intravitreal injection is indicated for the treatment of sympathetic ophthalmia, temporal arteritis, uveitis, and ocular inflammatory conditions. The intravitreal injection is also used for visualization during vitrectomy. An extended release suspension is indicated intra-articularly for management of pain in osteoarthritis of the knee.
A triamcinolone acetonide suspension for injection into the suprachoroidal space is indicated for the treatment of macular edema associated with uveitis.
What are the applications of Application
Triamcinolone is an inducer of apoptosis in T lymphocytes
Definition
ChEBI: A C21-steroid hormone that is 1,4-pregnadiene-3,20-dione carrying four hydroxy substituents at positions 11beta, 16alpha, 17alpha and 21 as well as a fluoro substituent at position 9. sed in the form of its 16,17-acetonide to treat various skin infections.
Indications
Intralesional bleomycin, a cytotoxic drug that inhibits DNA synthesis, is effective for all varieties of recalcitrant warts. Various concentrations of the drug have been used, although the total dose must be carefully tracked over time to avoid potential systemic toxicity.
Manufacturing Process
Preparation of δ1,4-Pregnandiene-9α-fluoro-11β,16α,17α,21-Tetrol-16,21-
Diacetate: An agar slant of Corynebacterium simplex was washed with 5 ml of
sterile saline and the spore suspension added to 100 ml of Trypticase soy
broth in a 500 ml Erlenmeyer. The mixture was incubated at 32°C for 8 hr and
1 ml was used to inoculate 10 flasks, each containing 100 ml of Trypticase soy
broth. The flasks were incubated with shaking at 32°C for 16 hr. 20 mg δ4-
pregnene-9α-fluoro-11β,16α,17α,21-tetrol-3,20-dione16,21-diacetate
dissolved in 2 ml ethanol was added and the flasks pooled. This solution was
extracted several times with methylene chloride, washed with saturated saline
and evaporated under reduced pressure. The residue was dissolved in
methanol, treated with activated charcoal, filtered through diatomaceous earth
and reevaporated to afford 277 mg of oil and acetylated overnight.
Paper strip chromatography showed approximately equal amounts of substrate
and a more polar product (δ1,4-pregnadiene-9α-fluoro-11β,16α,17α,21-tetrol-
3,20-dione 16,21-diacetate) together with very small amounts of two less
polar products. Partition chromatography of 0.25 gram of the residue
(diatomaceous earth column; system: 2 parts ethyl acetate, 3 parts petroleum
ether (90° to 100°C), 3 parts methanol and 2 parts water) separated the less
polar products and the substrate. The desired most polar product remained on
the column and was eluted with 500 ml of methanol. The residue (90 mg)
from the evaporated methanol was repartitioned on diatomaceous earth
[system: 3 parts ethyl acetate, 2 parts petroleum ether (90° to 100°C), 3
parts methanol, and 2 parts water] and the cut containing the desired product
(determined by ultraviolet absorption spectrum) was evaporated under
reduced pressure to afford 18 mg of solid.
Crystallization from acetone-petroleum ether gave 13 mg of colorless needles
of δ1,4-pregnadiene-9α-fluoro-11β,16α,17α,21-tetrol-3,20-dione16,21-
diacetate; melting point (Kofler block) about 150° to 240°C with apparent loss
of solvent at 150°C. Recrystallization from acetone-petroleum ether did not
alter the melting point.
Preparation of δ1,4-Pregnadiene-9α-Fluoro-11β,16α,17α,21-Tetrol-3,20-Dione:
A solution of 100 mg of δ1,4-pregnadiene-9α-fluoro-11β,16α,17α,21-tetrol-
3,20-dione16,21-diacetate was dissolved in 10 ml of methanol and cooled to
0°C. After flushing with nitrogen, a solution of 35 mg of potassium hydroxide
in 2 ml of methanol was added to the steroid solution. After standing at room
temperature for 1 hour, the solution was neutralized with glacial acetic acid
and evaporated under a nitrogen atmosphere to a white solid. Water was
added, and after cooling, the product was filtered and washed with water to
afford 52 mg of δ1,4-pregnadiene-9α-fluoro-11β,16α,17α,21-tetrol-3,20-dione,
melting point 246° to 249°C. Three crystallizations from acetone-petroleum
ether gave 29 mg of the tetrol, melting point 260° to 262.5°C according to US
Patent 2,789,118.
Therapeutic Function
Glucocorticoid
Biochem/physiol Actions
Triamcinolone is a synthetic glucocorticoid agonist; induces gene expression and apoptosis; inhibits prostaglandin synthesis; impairs tumor necrosis factor (TNF)-α-induced degradation of κB-α; potentiates the differentiation-inducing effects of bone morphogenetic proteins (BMP-2, -4, -6).
Pharmacokinetics
Triamcinolone is a corticosteroid with anti-inflammatory properties. These properties are used to treat inflammation in conditions that affect various organs and tissues. Triamcinolone should not be administered as an epidural injection.
Clinical Use
Triamcinolone to be used topically is generally dispensed as its more potent and lipophilic acetonide, a 16α,17α-methylenedioxy cyclic ketal or isopropylidene derivative. It is effective in the treatment of psoriasis and other corticoid-sensitive dermatologic conditions. Topically, triamcinolone acetonide is a more potent derivative of triamcinolone and is approximately eight times more active than prednisolone.
Side Effects
Even though triamcinolone has an apparently decreased tendency to cause salt and water retention and edema and may induce sodium and water diuresis, it causes other unwanted side effects, including anorexia, weight loss, muscle weakness, leg cramps, nausea, dizziness, and a general toxic feeling.
Safety Profile
Poison by subcutaneous route.An experimental teratogen. Other experimentalreproductive effects. Human mutation data reported.When heated to decomposition it emits toxic fumes of F??.An anti-inflammatory and antiallergic agent.
Synthesis
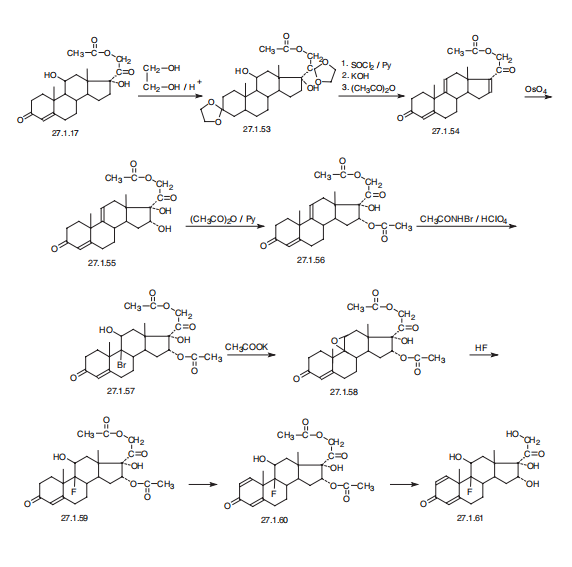
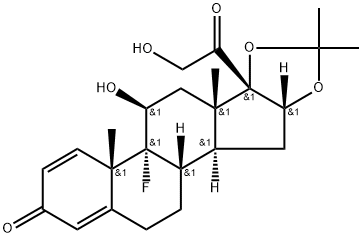
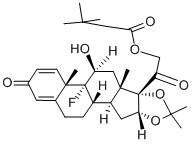
Triamcinolone, 9a-fluoro-11b,16a,17,21-tetrahydroxypregna-1, 4-dien-3,20-dione (27.1.61), differs from dexamethsone in terms of chemical structure in that the a methyl group at C16 is replaced with a hydroxyl group. It is synthesized from the 21-O-acetate of hydrocortisone 27.1.17. In the first stage, both carbonyl groups of this compound undergo ketalization by ethylene glycol. Next, the hydroxyl group in the resulting diketal 27.1.53 is replaced with chlorine using thionyl chloride, and the product undergoes dehydrochlorination using an alkaline, during which the 21-O-acetyl group also is hydrolyzed. Acetylating the hydroxyl group once again with acetic anhydride gives a triene 27.1.54. Reacting this with osmium tetroxide gives the vicinal diol 27.1.55. The secondary hydroxyl group at C16 of this product undergoes acetylation by acetic anhydride in pyridine, which forms the diacetate 27.1.56. Treating the product with N-bromoacetamide in chloric acid gives a bromohydrin (27.1.57), which upon reaction with potassium acetate is transformed to an epoxide (27.1.58). Opening of the epoxide ring, using hydrofluoric acid, gives the corresponding 9-fluoro-11-hydroxy derivative 27.1.59. Upon microbiological dehydrogenation, the C1¨CC2 bond is oxidized to a double bond, forming triamcinolone acetate (27.1.60), the acetyl group of which is hydrolyzed, forming the desired triamcinolone (27.1.61).
Drug interactions
Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use.
Antibacterials: metabolism accelerated by rifamycins;
metabolism possibly inhibited by erythromycin;
concentration of isoniazid possibly reduced.
Anticoagulants: efficacy of coumarins and
phenindione may be altered.
Antiepileptics: metabolism accelerated by
carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone.
Antifungals: increased risk of hypokalaemia with
amphotericin - avoid; metabolism possibly inhibited
by itraconazole and ketoconazole.
Antivirals: concentration possibly increased by
ritonavir.
Ciclosporin: rare reports of convulsions in patients
on ciclosporin and high-dose corticosteroids.
Cobicistat: concentration of triamcinolone possibly
increased.
Diuretics: enhanced hypokalaemic effects of
acetazolamide, loop diuretics and thiazide diuretics.
Vaccines: high dose corticosteroids can impair
immune response to vaccines; avoid with live
vaccines.
Metabolism
The major metabolite of triamcinolone is 6-beta-hydroxy-triamcinolone. Data regarding the metabolism of triamcinolone is not readily available.
Metabolism
Triamcinolone is metabolised largely hepatically but also by the kidney and is excreted in urine. The main metabolic route is 6-beta-hydroxylation; no significant hydrolytic cleavage of the acetonide occurs.
Properties of Triamcinolone
| Melting point: | 262-263 °C(lit.) |
| Boiling point: | 587.5±50.0 °C(Predicted) |
| alpha | 69 º (c=2, DMF) |
| Density | 1.1703 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMF: soluble20 mg/mL |
| form | neat |
| pka | 11.57±0.70(Predicted) |
| form | Solid |
| color | Crystals |
| optical activity | [α]25/D +69°, c = 2 in DMF |
| Water Solubility | 79.99mg/L(25 ºC) |
| Merck | 9595 |
| CAS DataBase Reference | 124-94-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Triamcinolone(124-94-7) |
| EPA Substance Registry System | Triamcinolone (124-94-7) |
Safety information for Triamcinolone
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Triamcinolone
| InChIKey | GFNANZIMVAIWHM-OBYCQNJPSA-N |
Triamcinolone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Triamcinolone 98%View Details
Triamcinolone 98%View Details -
 Triamcinolone 99%View Details
Triamcinolone 99%View Details -
 Triamcinolone 124-94-7 98%View Details
Triamcinolone 124-94-7 98%View Details
124-94-7 -
 124-94-7 Triamcinolone 98%View Details
124-94-7 Triamcinolone 98%View Details
124-94-7 -
 Triamcinolone 99%View Details
Triamcinolone 99%View Details
124-94-7 -
 Triamcinolone 124-94-7 99%View Details
Triamcinolone 124-94-7 99%View Details
124-94-7 -
 Triamcinolone 98% CAS 124-94-7View Details
Triamcinolone 98% CAS 124-94-7View Details
124-94-7 -
 Triamcinolone CAS 124-94-7View Details
Triamcinolone CAS 124-94-7View Details
124-94-7
