Trenbolone acetate
Synonym(s):17β-Hydroxyestra-4,9,11-trien-3-one acetate
- CAS NO.:10161-34-9
- Empirical Formula: C20H24O3
- Molecular Weight: 312.4
- MDL number: MFCD00214396
- EINECS: 233-432-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-24 17:44:56
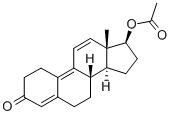
What is Trenbolone acetate?
Description
Trenbolone acetate (brand name: Revalor) is a 19-nortestosterone derivative and a synthetic, injected anabolic-androgenic steroid that is used in veterinary medicine. It is a highly efficient anabolic steroid that can greatly enhance the performance of athletes. Trenbolone acetate has several physiological effects. First, it can greatly promote insulin-like growth factor-1 (IGF-1). IGF-1 is a powerful and natural anabolic hormone that of great importance for muscle tissue development. Secondly, it can greatly increase the red blood cell count, further increasing blood oxygen and enhancing muscular endurance. Moreover, it can inhibit the glucocorticoid hormone, a hormone that destroy muscle tissue and promote fat. Finally, it can improve nutrient uptake and consumption efficiency.
Chemical properties
Pale Yellow Solid
Originator
Parabolan,Negma,France,1980
The Uses of Trenbolone acetate
A steroid used to increase the muscle growth of livestock.
The Uses of Trenbolone acetate
anti-cataract agent
Definition
ChEBI: Trenbolone acetate is a steroid ester.
Manufacturing Process
Stage A: Preparation of 17β-Benzoyloxy-Estra-4,9,11-Trien-3-one - 0.400 g of
17β-benzoyloxy-4,5-seco-estra-9,11-diene-3,5-dione is dissolved in 4 cc of
toluene under an inert atmosphere. The solution is cooled to 3°C, then 0.48
cc is added of the solution of sodium tert-amylate in anhydrous toluene,
diluted by the addition of a further 4.8 cc of anhydrous toluene.
This reaction mixture is kept between 0°C and +5°C for six hours, with
agitation and under an inert atmosphere, then 5 cc of a 0.2 N solution of
acetic acid in toluene are added. The mixture is extracted with toluene, and
the extracts are washed with water and evaporated to dryness. The residue is
taken up in ethyl acetate, and then the solution is evaporated to dryness in
vacuo, yielding a resin which is dissolved in methylene chloride, and the
solution passed through a column of 40 g of magnesium silicate. Elution is
carried out first with methylene chloride, then with methylene chloride
containing 0.5% of acetone, and 0.361 g is thus recovered of a crude product,
which is dissolved in 1.5 cc of isopropyl ether; then hot methanol is added
and the mixture left at 0°C for one night.
0.324 g of the desired 17β-benzoyloxy-estra-4,9,11-trien-3-one are thus
finally obtained, being a yield of 85%, melting point is 154°C.
Stage B: Preparation of 17β-Hydroxy-Estra-4,9,11-Trien-3-one - 3 g of 17β-
benzoyloxy-estra-4,9,11-trien-3-one, obtained as described in Stage A are
dissolved in 15 cc of methanol. 0.03 g of hydroquinone is added, and the
mixture is taken to reflux while bubbling in nitrogen. Then 1.2 cc of 11%
methanolic caustic potash is added and reflux is maintained for three hours,
after which the reaction product is acidified with 0.36 cc of acetic acid.
The methyl benzoate thus formed is eliminated by steam distillation, and
2.140 g of crude product are obtained, which are dissolved in 20 cc of
methylene chloride. This solution is passed through 10 parts of magnesium
silicate, elution being performed with 250 cc of methylene chloride containing
5% of acetone. After evaporation of the solvent 2.050 g of product is
recovered, which is recrystallized from isopropyl ether.
In this way 1.930 g of the desired 17β-hydroxy-estra-4,9,11-trien-3-one is
obtained being a yield of 89%, melting point is 186°C. It is converted to the
acetate by acetic anhydride.
brand name
[Trenbolone is INN and BAN.
Therapeutic Function
Anabolic steroid
References
https://en.wikipedia.org/wiki/Trenbolone_acetate
Henricks, D. M., et al. "Serum concentrations of trenbolone-17 beta and estradiol-17 beta and performance of heifers treated with trenbolone acetate, melengestrol acetate, or estradiol-17 beta." Journal of Animal Science 75.10(1997):2627-33.
https://www.steroid.com/trenbolone-acetate.php
Properties of Trenbolone acetate
| Melting point: | 90-92°C |
| alpha | D20 +36.8° (c = 0.37 in methanol) |
| Boiling point: | 392.32°C (rough estimate) |
| Density | 1.1464 (rough estimate) |
| refractive index | 1.4618 (estimate) |
| storage temp. | 0-6°C |
| solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; DMSO:PBS(pH 7.2) (1:3): 0.25 mg/ml; Ethanol: 10 mg/ml |
| form | neat |
| InChI | InChI=1/C20H24O3/c1-12(21)23-19-8-7-18-17-5-3-13-11-14(22)4-6-15(13)16(17)9-10-20(18,19)2/h9-11,17-19H,3-8H2,1-2H3/t17-,18+,19+,20+/s3 |
| CAS DataBase Reference | 10161-34-9(CAS DataBase Reference) |
| EPA Substance Registry System | Trenbolone acetate (10161-34-9) |
Safety information for Trenbolone acetate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Trenbolone acetate
| InChIKey | CMRJPMODSSEAPL-FYQPLNBISA-N |
| SMILES | [C@@]12([H])CC[C@H](OC(=O)C)[C@@]1(C)C=CC1=C3CCC(=O)C=C3CC[C@@]21[H] |&1:0,4,9,23,r| |
Trenbolone acetate manufacturer
Omicron Pharmatech Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 buytrenbolone Acetate USA UK Delivery in INDIA, USPView Details
buytrenbolone Acetate USA UK Delivery in INDIA, USPView Details
10161-34-9 -
 Evolve Biolabs Trenbolone Acetate 100 Mg/ml, Packaging Type: BoxView Details
Evolve Biolabs Trenbolone Acetate 100 Mg/ml, Packaging Type: BoxView Details
10161-34-9 -
 BUY FREE SHIPPING Trenbolone Acetate IN INDIA IN FLORIDAView Details
BUY FREE SHIPPING Trenbolone Acetate IN INDIA IN FLORIDAView Details
10161-34-9 -
 Trenbolone Acetate 200 Mg, For Muscle Building, 10 Ampules Of 1mlView Details
Trenbolone Acetate 200 Mg, For Muscle Building, 10 Ampules Of 1mlView Details
10161-34-9 -
 Trenbolone Acetate 100 MgView Details
Trenbolone Acetate 100 MgView Details
10161-34-9 -
 EVOLVE BIOLABS Trenbolone Acetate 100 Mg, Packaging Type: VialView Details
EVOLVE BIOLABS Trenbolone Acetate 100 Mg, Packaging Type: VialView Details
10161-34-9 -
 Trenbolone Acetate PowderView Details
Trenbolone Acetate PowderView Details
10161-34-9 -
 10161-34-9 98%View Details
10161-34-9 98%View Details
10161-34-9
