Trenbolone
Synonym(s):17β-Hydroxyestra-4,9,11-trien-3-one
- CAS NO.:10161-33-8
- Empirical Formula: C18H22O2
- Molecular Weight: 270.37
- MDL number: MFCD00214395
- EINECS: 600-229-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
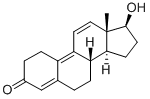
What is Trenbolone?
Description
Trenbolone, also known as trienolone or trienbolone, is a kind of anabolic androgenic steroid belonging to the 19-nortestosterone group. It can be supplied to veterinary medicine of livestock in order the boost muscle growth and appetite. It can also enhance the performance of athletes. It has several physiological effects: (1) enhance protein synthesis and nitrogen retention in the muscle tissues, which boost enhanced anabolism; (2) greatly promote insulin-like growth factor-1 (IGF-1) which is a highly effective anabolic hormone; (3) Greatly increase the red blood cell count to boost blood oxygenation which leads to improved muscular endurance; (4) Inhibit glucocorticoid hormones which inhibit the growth of muscle tissue and promote fat. (5) Improve food efficiency.
Description
17β-Trenbolone is an anabolic steroid synthesized in 1963 by L. Velluz and co-workers. Veterinarians use it, most commonly as an ester, to increase muscle growth and appetite in livestock. It has also been illicitly used by athletes as an adjunct to weight training to build body mass.
When steroids such as 17β-trenbolone enter water supplies, they may disrupt the endocrine systems of wildlife that drink the water. Until recently, it was thought that the compounds decompose too quickly to pose a serious threat. But D. M. Cwiertny at the University of Iowa and E. P. Kolodziej at the University of Nevada, Reno, report that?these steroids are degraded by light but re-form in the dark. Their findings suggest that steroid contaminants should be included in environmental risk assessment.
?
Chemical properties
Yellow Solid
Originator
Parabolan,Negma,France,1980
The Uses of Trenbolone
Trenbolone is a controlled substance (anabolic steroid). It is a steroid used on livestock to increase muscle growth and appetite. To increase its effective half-life, trenbolone is administered as a prodrug as an ester conjugate such as [trenbolone acetate], trenbolone enanthate, or trenbolone cyclohexylmethylcarbonate. Plasma lipases then cleave the ester group in the bloodstream leaving free trenbolone.
Definition
ChEBI: Trenbolone is a 3-oxo-Delta(4) steroid that is estra-4,9,11-triene carrying an oxo group at position 3 and a hydroxy group at position 17beta. It is a synthetic anabolic steroid used for muscle growth in livestock. It has a role as a plant metabolite and an endocrine disruptor. It is a 3-oxo-Delta(4) steroid, a 17beta-hydroxy steroid, a C18-steroid and an anabolic androgenic steroid.
Preparation
Trenbolone synthesis: To a cold mixture of 47.9 g 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in 375 mL anhydrous methylene chloride (DCM), a solution of 54.9 g compound (II-a) and 11.5 mL acetic acid in 150 mL anhydrous DCM is added dropwise under stirring, while keeping the internal temperature at -5 °C. The addition funnel is rinsed with 25 mL DCM and this aliquot is added to the reaction mixture. After 4 hours, reaction conversion reaches 99.25% by HPLC. The reaction mixture is quenched by addition of a solution composed of 5.7 g of Na2S2O5, 50 mL water and 10 mL MeOH. The obtained slurry is warmed to 15-23°C and left stirring for 0.5 h, after which it was filtered. The filter cake is washed with 2x40 mL DCM and the solid discarded. The obtained biphasic mixture is separated, and the organic phase washed twice with a solution of 100 mL water, 10 mL MeOH and 3.9 g of NaHCO3. The phases are separated, and the organic layer washed one final time with a solution of 100 mL water, 10 mL MeOH and 3.9 g of NaHCO3. The combined solution is concentrated with stirring to 150 mL (total volume) under vacuum, keeping the internal temperature below 30 °C. To the resulting solution, 150 mL of acetone are added, and the obtained mixture concentrated under vacuum to 150 mL (total volume) again, keeping the internal temperature below 30 °C. This addition/concentration protocol is repeated 3 times. The resulting suspension is cooled to 0 °C and kept at this temperature for 0.5 h with stirring, after which it is filtered, and the filter cake is washed twice with 50 mL cold acetone. The wet solid is dried at 40 °C under vacuum to give Trenbolone in 76% yield and 98.2 % A/A purity by HPLC.
Synthesis of Trenbolone acetate
brand name
[Trenbolone is INN and BAN.
Therapeutic Function
Anabolic steroid
Side Effects
Trenbolone's side effects aren't only physical but also mental, with users commonly reporting feeling increasingly: irritable, anxious, paranoid and depressed (than on other steroids).
Such side effects can be linked to Trenbolone having a stimulating effect on the central nervous system, causing an increase in adrenaline output and thus shifting Tren-users into a state of fight or flight mode.
Safety Profile
When heated to decomposition it emits acrid smoke and irritating fumes.
References
https://en.wikipedia.org/wiki/Trenbolone
https://www.steroid.com/Trenbolone.php
Škorjanc, D, M. Brus, and I. Vojtic. "A short review of chain controlling systems in livestock production technology. " Agricultura (2005).
Properties of Trenbolone
| Melting point: | 170°C |
| Boiling point: | 490.8±45.0 °C(Predicted) |
| alpha | D20 +19° (c = 0.45 in ethanol) |
| Density | 1.19±0.1 g/cm3(Predicted) |
| Flash point: | 2℃ |
| storage temp. | 2-8°C |
| solubility | DMF: 30 mg/ml; DMF:PBS (pH 7.2)(1:3): 0.25 mg/ml; DMSO: 20 mg/ml; Ethanol: 2 mg/ml |
| form | neat |
| pka | 14.73±0.40(Predicted) |
| InChI | InChI=1/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h8-10,15-17,20H,2-7H2,1H3/t15-,16+,17+,18+/s3 |
| CAS DataBase Reference | 10161-33-8(CAS DataBase Reference) |
| EPA Substance Registry System | Estra-4,9,11-trien-3-one, 17-hydroxy-, (17.beta)- (10161-33-8) |
Safety information for Trenbolone
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Trenbolone
| InChIKey | MEHHPFQKXOUFFV-OWSLCNJRSA-N |
| SMILES | [C@@]12([H])CC[C@H](O)[C@@]1(C)C=CC1=C3CCC(=O)C=C3CC[C@@]21[H] |&1:0,4,6,20,r| |
Trenbolone manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1